question_answer 1) The position of the particle moving along Y-axis is given as \[y=A{{t}^{2}}-B{{t}^{3}},\] where y is measured in metre and t in second. Then the dimensions of B is
A)
\[[L{{T}^{-2}}]\]
done
clear
B)
\[[L{{T}^{-1}}]\]
done
clear
C)
\[[L{{T}^{-3}}]\]
done
clear
D)
\[[ML{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 2) A conveyor belt is moving horizontally at a speed of 4 m/s. A box of mass 20 kg is gently laid on it. It takes 0.1s for the box to come to rest. If the belt continues to move uniformly, then the distance moved by the box on the conveyor belt is
A)
zero
done
clear
B)
0.2 m
done
clear
C)
0.4 m
done
clear
D)
0.8 m
done
clear
View Answer play_arrow
question_answer 3) An open knife edge of mass m is dropped from a height h on a wooden floor. If the blade penetrates s into the wood the average resistance offered by the wood to the blade is
A)
\[Mg\]
done
clear
B)
\[Mg\left( \frac{h}{s} \right)\]
done
clear
C)
\[Mg\left( 1-\frac{h}{s} \right)\]
done
clear
D)
\[Mg{{\left( 1+\frac{h}{s} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 4) Energy required to accelerate a car from 10 m/s to 20 m/s compared with that required to accelerate it from 0 to 10 m/s is
A)
twice
done
clear
B)
thrice
done
clear
C)
four times
done
clear
D)
same
done
clear
View Answer play_arrow
question_answer 5) A solid sphere of mass 2 kg rolls up a \[30{}^\circ \] incline with an initial speed of 10 m/s. The maximum height reached by the sphere is \[(g=10\,m\text{/}{{s}^{2}})\]
A)
3.5 m
done
clear
B)
7.0 m
done
clear
C)
10.5 m
done
clear
D)
14.0 m
done
clear
View Answer play_arrow
question_answer 6) Two satellites are moving in the same circular orbit around the earth. They must have the same
A)
mass
done
clear
B)
angular momentum
done
clear
C)
kinetic energy
done
clear
D)
speed
done
clear
View Answer play_arrow
question_answer 7) A planet having average surface temperature \[{{T}_{0}}\]at an average distance d from the sun. Assuming that the planet receives radiant energy from the sun only and it loses radiant energy only from the surface and neglecting all other atmospheric effects we conclude
A)
\[{{T}_{0}}\propto {{d}^{2}}\]
done
clear
B)
\[{{T}_{0}}\propto {{d}^{-2}}\]
done
clear
C)
\[{{T}_{0}}\propto {{d}^{1/2}}\]
done
clear
D)
\[{{T}_{0}}\propto {{d}^{-1/2}}\]
done
clear
View Answer play_arrow
question_answer 8) Time period of a simple pendulum is T. If its length increases by 2%, the new time period becomes
A)
0.98 T
done
clear
B)
1.02 T
done
clear
C)
0.99 T
done
clear
D)
1.01 T
done
clear
View Answer play_arrow
question_answer 9) A boat of anchor is rocket by waves of velocity 25 m/s having crests 100 m apart. They reach the boat once every
A)
4.0 s
done
clear
B)
8.0 s
done
clear
C)
2.0 s
done
clear
D)
0.25 s
done
clear
View Answer play_arrow
question_answer 10) Two point charges +2C and +6C repel each other with a force of 12 N. If a charge of -2C is given to each of these charges the force will now be
A)
zero
done
clear
B)
8 N (attractive)
done
clear
C)
8 N (repulsive)
done
clear
D)
None of these
done
clear
View Answer play_arrow
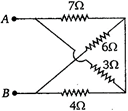
question_answer 11)
The equivalent resistance between A and B (of the circuit shown) is
A)
4.5\[\Omega \]
done
clear
B)
12\[\Omega \]
done
clear
C)
5.4\[\Omega \]
done
clear
D)
20\[\Omega \]
done
clear
View Answer play_arrow
question_answer 12) A 2 kW boiler used for 1 h/day consumes the following electrical energy in thirty days
A)
60 unit
done
clear
B)
120 unit
done
clear
C)
15 unit
done
clear
D)
\[6\times {{10}^{4}}\]unit
done
clear
View Answer play_arrow
question_answer 13) Two long straight wires are set parallel to each other at separation r and each carries a current I in the same direction. The strength of the magnetic field at any point midway between the two wires is
A)
\[\frac{{{\mu }_{0}}I}{\pi r}\]
done
clear
B)
\[\frac{2{{\mu }_{0}}I}{\pi r}\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{2\pi r}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 14) A inductive coil has a resistance of 100\[\Omega \]. When an AC signal of frequency 1000 Hz is applied to the coil, the voltage leads the current by\[45{}^\circ \]. The inductance of the coil is
A)
\[\frac{1}{10\pi }\]
done
clear
B)
\[\frac{1}{20\pi }\]
done
clear
C)
\[\frac{1}{40\pi }\]
done
clear
D)
\[\frac{1}{60\pi }\]
done
clear
View Answer play_arrow
question_answer 15) A boy stands straight in front of a mirror at a distance of 30 cm from it. He sees his erect image whose height is\[\frac{1}{5}\]of his real height. The mirror he is using, is
A)
plane
done
clear
B)
convex
done
clear
C)
concave
done
clear
D)
plano-concave
done
clear
View Answer play_arrow
question_answer 16) In the depletion region of an unbiased p-n junction diode, there are
A)
only electrons
done
clear
B)
only holes
done
clear
C)
both electrons and holes
done
clear
D)
only fixed ions
done
clear
View Answer play_arrow
question_answer 17) The condition under which vectors \[(\overrightarrow{a}+\overrightarrow{b})\] and \[(\overrightarrow{a}-\overrightarrow{b})\] should be at right angles to each other is
A)
\[\overrightarrow{a}\ne \overrightarrow{b}\]
done
clear
B)
\[\overrightarrow{a}\cdot \overrightarrow{b}=0\]
done
clear
C)
\[|\overrightarrow{a}|\,\,=\,\,|\overrightarrow{b}|\]
done
clear
D)
\[\overrightarrow{a}\cdot \overrightarrow{b}=1\]
done
clear
View Answer play_arrow
question_answer 18) The error in the measurement of radius of the sphere is 0.3%. What is the permissible error in its surface area?
A)
0.6%
done
clear
B)
1.2%
done
clear
C)
1.8%
done
clear
D)
2.4%
done
clear
View Answer play_arrow
question_answer 19) A gas is heated at constant pressure. The fraction of heat supplied used for external work is
A)
\[\frac{1}{\gamma }\]
done
clear
B)
\[\left( 1-\frac{1}{\gamma } \right)\]
done
clear
C)
\[\gamma -1\]
done
clear
D)
\[\left( 1-\frac{1}{{{\gamma }^{2}}} \right)\]
done
clear
View Answer play_arrow
question_answer 20) The maximum displacement of the particle executing SHM is 1 cm and the maximum acceleration is\[{{(1.57)}^{2}}cm\text{/}{{s}^{2}}.\]Its time period is
A)
0.25 s
done
clear
B)
4.0 s
done
clear
C)
1.57 s
done
clear
D)
3.14 s
done
clear
View Answer play_arrow
question_answer 21) The equation of a wave travelling on a string is \[y=4\,\sin \left[ \frac{\pi }{2}\left( 8t-\frac{x}{8} \right) \right],\] where x, y are in cm and t in second. The velocity of the wave is
A)
64 cm/s, in - x direction
done
clear
B)
32 cm/s, in - x direction
done
clear
C)
32 cm/s, in + x direction
done
clear
D)
64 cm/s, in + x direction
done
clear
View Answer play_arrow
question_answer 22)
In the figure, the equivalent capacitance between A and B is
A)
3.75\[\mu F\]
done
clear
B)
5.25\[\mu F\]
done
clear
C)
6.5\[\mu F\]
done
clear
D)
10.5\[\mu F\]
done
clear
View Answer play_arrow
question_answer 23) Two bulbs 100 W, 250 V and 200 W, 250 V are connected in parallel across a 500 V line. Then
A)
100 W bulb will be fused
done
clear
B)
200 W bulb will be fused
done
clear
C)
both bulbs will be fused
done
clear
D)
no bulb will be fused
done
clear
View Answer play_arrow
question_answer 24) A long solenoid has 20 turns/cm. The current necessary to produce a magnetic field of 20 mT inside the solenoid is approximately
A)
1 A
done
clear
B)
2 A
done
clear
C)
4 A
done
clear
D)
8 A
done
clear
View Answer play_arrow
question_answer 25) The inductance of a coil is proportional to
A)
its length
done
clear
B)
the number of turns
done
clear
C)
the resistance of the coil
done
clear
D)
the square of the number of turns
done
clear
View Answer play_arrow
question_answer 26) A photosensitive surface is receiving light of wavelength 5000\[\overset{\text{o}}{\mathop{\text{A}}}\,\] at the rate of\[{{10}^{-7}}\,J\text{/}s.\]. The number of photons ejected per second is
A)
\[2.5\times {{10}^{12}}\]
done
clear
B)
\[2.5\times {{10}^{11}}\]
done
clear
C)
\[2.5\times {{10}^{10}}\]
done
clear
D)
\[2.5\times {{10}^{9}}\]
done
clear
View Answer play_arrow
question_answer 27) Two discs have same mass and same thickness. Their materials are of densities \[{{\rho }_{1}}\] and\[{{\rho }_{2}}\]. The ratio of their moments of inertia about central axis will be
A)
\[{{\rho }_{1}}{{\rho }_{2}}:1\]
done
clear
B)
\[1:{{\rho }_{1}}{{\rho }_{2}}\]
done
clear
C)
\[{{\rho }_{1}}:{{\rho }_{2}}\]
done
clear
D)
\[{{\rho }_{2}}:{{\rho }_{1}}\]
done
clear
View Answer play_arrow
question_answer 28) The Youngs modulus of the material of the wire of length \[L\] and radius r is\[Y\,N\text{/}{{m}^{2}}\]. If the length is reduced to \[L\text{/}2\] and radius r/2, the Youngs modulus will be
A)
Y/2
done
clear
B)
Y
done
clear
C)
2Y
done
clear
D)
4Y
done
clear
View Answer play_arrow
question_answer 29) A body floats with one-third of its volume outside water and 3/4 of its volume outside another liquid. The density of the other liquid is
A)
\[\frac{9}{4}g\text{/}cc\]
done
clear
B)
\[\frac{4}{0}g\text{/}cc\]
done
clear
C)
\[\frac{8}{3}g\text{/}cc\]
done
clear
D)
\[\frac{3}{8}g\text{/}cc\]
done
clear
View Answer play_arrow
question_answer 30) The terminal velocity of small-sized spherical body of radius r falling vertically in a viscous liquid is given by the following proportionality
A)
\[1\text{/}{{r}^{2}}\]
done
clear
B)
\[1\text{/}r\]
done
clear
C)
\[r\]
done
clear
D)
\[{{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 31) The reading of a manometer fitted to a closed tap is \[3.5\times {{10}^{5}}N\text{/}{{m}^{2}}.\] If the valve is opened, the reading of the manometer falls to \[3.5\times {{10}^{5}}N\text{/}{{m}^{2}}.\] The velocity of water is
A)
1 m/s
done
clear
B)
10 m/s
done
clear
C)
100 m/s
done
clear
D)
0.1 m/s
done
clear
View Answer play_arrow
question_answer 32) One litre of an ideal gas at \[27{}^\circ C\] is heated at a constant pressure to\[297{}^\circ C\]. Then, the final volume is approximately
A)
1.2 L
done
clear
B)
1.9 L
done
clear
C)
19 L
done
clear
D)
2.4 L
done
clear
View Answer play_arrow
question_answer 33) Coefficient of cubical expansion of water is zero at
A)
\[0{}^\circ C\]
done
clear
B)
\[4{}^\circ C\]
done
clear
C)
\[15.5{}^\circ C\]
done
clear
D)
\[100{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 34) A cylindrical conductor is placed near another. positively charged conductor; The net charge acquired by the cylindrical conductor will be
A)
positive only
done
clear
B)
negative only
done
clear
C)
zero
done
clear
D)
either positive or negative
done
clear
View Answer play_arrow
question_answer 35) The ratio of areas between the electron orbits for the first excited state to the ground state for the hydrogen atom is
A)
2 : 1
done
clear
B)
4 : 1
done
clear
C)
8 : 1
done
clear
D)
16 : 1
done
clear
View Answer play_arrow
question_answer 36) An electron, accelerated by a potential difference V, has de-Broglie wavelength\[\lambda .\] If the electron is accelerated by a potential difference 4V, its de-Broglie wavelength will be
A)
\[2\lambda \]
done
clear
B)
\[4\lambda \]
done
clear
C)
\[\lambda \text{/}2\]
done
clear
D)
\[\lambda \text{/}4\]
done
clear
View Answer play_arrow
question_answer 37) A convex lens of focal length 40 cm is in contact with a concave lens of focal length 25 cm. The power of the combination in dioptre is
A)
- 1.5
done
clear
B)
- 6.5
done
clear
C)
+ 6.5
done
clear
D)
+ 6.67
done
clear
View Answer play_arrow
question_answer 38) A material particle with a rest mass \[{{m}_{0}}\] is moving with speed of light c. The de-Broglie wavelength associated is given by
A)
\[\frac{h}{{{m}_{0}}c}\]
done
clear
B)
\[\frac{{{m}_{0}}c}{h}\]
done
clear
C)
zero
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 39) The impedance of a circuit consists of \[3\Omega \] resistance and \[4\Omega \] resistance. The power factor of the circuit is
A)
0.4
done
clear
B)
0.6
done
clear
C)
0.8
done
clear
D)
1.0
done
clear
View Answer play_arrow
question_answer 40) A glass has refractive index \[\frac{3}{2}\] and water has refractive index\[\frac{4}{3}\]. If the speed of light in glass is \[2\times {{10}^{8}}m\text{/}s,\] the speed of light in water in m/s is
A)
\[1.5\times {{10}^{6}}\]
done
clear
B)
\[1.78\times {{10}^{8}}\]
done
clear
C)
\[2.25\times {{10}^{8}}\]
done
clear
D)
\[2.67\times {{10}^{8}}\]
done
clear
View Answer play_arrow
question_answer 41) A bulb is placed between two plane mirrors inclined at an angle of \[60{}^\circ \]. The number of images formed is
A)
5
done
clear
B)
6
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 42) A piece of semiconductor is connected in series in an electric circuit. On increasing the temperature, the current in the circuit will
A)
decrease
done
clear
B)
remain unchanged
done
clear
C)
increase
done
clear
D)
stop flowing
done
clear
View Answer play_arrow
question_answer 43) If the wavelength of the first line of the Balmer series of hydrogen is 6561\[\overset{\text{o}}{\mathop{\text{A}}}\,\], the wavelength of the second line of the series should be
A)
13122 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
3280 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
4860 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
2187 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 44) Activity of a radioactive element decreased to one third of original activity \[{{R}_{0}}\] in 9 yr. After further 9 yr, its activity will be
A)
\[{{R}_{0}}\]
done
clear
B)
\[\frac{2}{3}{{R}_{0}}\]
done
clear
C)
\[\frac{{{R}_{0}}}{9}\]
done
clear
D)
\[\frac{{{R}_{0}}}{6}\]
done
clear
View Answer play_arrow
question_answer 45) In a potentiometer experiment, the galvano-meter shows no deflection when a cell is connected across 60 cm of the potentiometer wire. If the cell is shunted by a resistance of 6\[\Omega \], the balance is obtained across 50 cm of the wire. The internal resistance of the cell is
A)
0.50
done
clear
B)
0.60
done
clear
C)
1.20
done
clear
D)
1.50
done
clear
View Answer play_arrow
question_answer 46) A nuclear reaction given by \[{}_{Z}{{X}^{A}}\xrightarrow{{}}{}_{Z+1}{{Y}^{A}}+{}_{-1}{{e}^{0}}+\bar{v}\]represents.
A)
\[\gamma \]-decay
done
clear
B)
fusion
done
clear
C)
fission
done
clear
D)
\[\beta \]-decay
done
clear
View Answer play_arrow
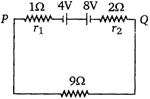
question_answer 47)
Two batteries of emf 4 V and 8 V with internal resistance 1\[\Omega \] and 2\[\Omega \] are connected in a circuit with a resistance of 9\[\Omega \] as shown in figure. The current and potential difference between the points P and Q are
A)
\[\frac{1}{3}A\]and 3 V
done
clear
B)
\[\frac{1}{6}A\] and 4V
done
clear
C)
\[\frac{1}{9}A\]and 9 V
done
clear
D)
\[\frac{1}{2}A\]and 12 V
done
clear
View Answer play_arrow
question_answer 48) Light of wavelength 4000\[\overset{\text{o}}{\mathop{\text{A}}}\,\] is incident on a sodium surface for which the threshold wavelength of photoelectron is 5420\[\overset{\text{o}}{\mathop{\text{A}}}\,\]. The work function of sodium is
A)
4.58 eV
done
clear
B)
2.28 eV
done
clear
C)
1.14eV
done
clear
D)
0.57 eV
done
clear
View Answer play_arrow
question_answer 49) In a Youngs double slit experiment, the slit separation is 1 mm and the screen in 1 m from the slit. For a monochromatic light of wavelength 500 nm, the distance of 3rd minima from central maxima is
A)
0.50 mm
done
clear
B)
1.25 mm
done
clear
C)
1.50mm
done
clear
D)
1.75mm
done
clear
View Answer play_arrow
question_answer 50) In order to double the frequency of the fundamental note emitted by a stretched string, the length is reduced to \[\frac{3}{4}\]th of the original length and the tension is changed. The factor by which the tension is to be changed, is
A)
\[\frac{3}{8}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[\frac{8}{9}\]
done
clear
D)
\[\frac{9}{4}\]
done
clear
View Answer play_arrow
question_answer 51) In a given atom no two electrons can have the same values for all the four quantum numbers. This is called
A)
Hunds rule
done
clear
B)
Aufbaus principle
done
clear
C)
Uncertainty principle
done
clear
D)
Paulis exclusion principle
done
clear
View Answer play_arrow
question_answer 52) For reaction, \[2NOCl(g)\rightleftharpoons 2NO(g)+C{{l}_{2}}(g),\]\[{{K}_{c}}\]at \[427{}^\circ C\] is \[3\times {{10}^{-6}}L\,\,\text{mo}{{\text{l}}^{-1}}\]. The value of \[{{K}_{p}}\]is nearly
A)
\[7.5\times {{10}^{-5}}\]
done
clear
B)
\[2.5\times {{10}^{-5}}\]
done
clear
C)
\[2.5\times {{10}^{-4}}\]
done
clear
D)
\[1.72\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 53) What is the order of a reaction which has a rate expression, rate\[=k{{(A)}^{3/2}}{{(B)}^{-1}}?\]
A)
3/2
done
clear
B)
½
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 54) Which of th following compounds is highest covalent?
A)
\[LiCl\]
done
clear
B)
\[LiF\]
done
clear
C)
\[LiBr\]
done
clear
D)
\[LiI\]
done
clear
View Answer play_arrow
question_answer 55) Shape of\[Xe{{F}_{4}}\]molecule is
A)
linear
done
clear
B)
pyramidal
done
clear
C)
tetrahedral
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 56) Which one of the following elements has the highest ionisation energy?
A)
\[[Ne]\,3{{s}^{2}}3{{p}^{1}}\]
done
clear
B)
\[[Ne]\,3{{s}^{2}}3{{p}^{2}}\]
done
clear
C)
\[[Ne]\,3{{s}^{2}}3{{p}^{3}}\]
done
clear
D)
\[[Ar]\,3{{d}^{10}},\,4{{s}^{2}}4{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 57) Important ore of zinc is
A)
calamine
done
clear
B)
cryolite
done
clear
C)
cassiterite
done
clear
D)
malachite
done
clear
View Answer play_arrow
question_answer 58) Nesslers reagent is
A)
\[KHg{{I}_{4}}\]
done
clear
B)
\[{{K}_{2}}Hg{{I}_{4}}+N{{H}_{4}}OH\]
done
clear
C)
\[{{K}_{2}}Hg{{I}_{4}}+KOH\]
done
clear
D)
\[KHg{{I}_{4}}+N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 59) Potassium is kept in
A)
alcohol
done
clear
B)
water
done
clear
C)
kerosene
done
clear
D)
liquid ammonia
done
clear
View Answer play_arrow
question_answer 60) Which of the following oxides of nitrogen is the anhydride of nitrous acid?
A)
\[NO\]
done
clear
B)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 61) Which one of the given below is a pseudohalide?
A)
\[C{{N}^{-}}\]
done
clear
B)
\[ICl\]
done
clear
C)
\[I{{F}_{5}}\]
done
clear
D)
\[I_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 62) Acidified potassium permanganate solution is decolourised by
A)
bleaching powder
done
clear
B)
white vitriol
done
clear
C)
Mohrs salt
done
clear
D)
microcosmic salt
done
clear
View Answer play_arrow
question_answer 63) The pressure and temperature of 4\[d{{m}^{3}}\] of carbon dioxide gas are doubled. Then, volume of carbon dioxide gas would be
A)
2\[d{{m}^{3}}\]
done
clear
B)
3\[d{{m}^{3}}\]
done
clear
C)
4\[d{{m}^{3}}\]
done
clear
D)
8\[d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 64) An fee unit cell of aluminium contains the equivalent of how many atoms?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 65) Hesss law deals with
A)
changes in heat of reaction
done
clear
B)
rate of reaction
done
clear
C)
equilibrium constant
done
clear
D)
influence of pressure on volume of gas
done
clear
View Answer play_arrow
question_answer 66) Hydrolysis of sodium acetate will give
A)
acidic solution
done
clear
B)
basic solution
done
clear
C)
neutral solution
done
clear
D)
normal solution
done
clear
View Answer play_arrow
question_answer 67) Which of the following is not a non- electrolyte?
A)
Acetic acid
done
clear
B)
Glucose
done
clear
C)
Ethanol
done
clear
D)
Urea
done
clear
View Answer play_arrow
question_answer 68) The oxidation state of S in\[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] is
A)
+ 2
done
clear
B)
+ 4
done
clear
C)
+ 6
done
clear
D)
+ 7
done
clear
View Answer play_arrow
question_answer 69) Which of the following free radicals is most stable?
A)
Primary
done
clear
B)
Methyl
done
clear
C)
Secondary
done
clear
D)
Tertiary
done
clear
View Answer play_arrow
question_answer 70) Which of the following has highest knocking property?
A)
Aromatic hydrocarbons
done
clear
B)
Olefins
done
clear
C)
Branched chain paraffins
done
clear
D)
Straight chain paraffins
done
clear
View Answer play_arrow
question_answer 71) Which of the following is the most stable alkene?
A)
\[{{R}_{2}}C=C{{R}_{2}}\]
done
clear
B)
\[RCH=CHR\]
done
clear
C)
\[RCH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) From Williamsons synthesis preparation of which of the following is possible?
A)
Only symmetrical ethers
done
clear
B)
Only asymmetrical ethers
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 73) Dry heating of calcium acetate gives
A)
acetaldehyde
done
clear
B)
ethane
done
clear
C)
acetic acid
done
clear
D)
acetone
done
clear
View Answer play_arrow
question_answer 74) Formaldehyde reacts with ammonia to give urotropine that is
A)
\[{{(C{{H}_{2}})}_{6}}{{N}_{4}}\]
done
clear
B)
\[{{(C{{H}_{2}})}_{4}}{{N}_{3}}\]
done
clear
C)
\[{{(C{{H}_{2}})}_{6}}{{N}_{6}}\]
done
clear
D)
\[{{(C{{H}_{2}})}_{3}}{{N}_{3}}\]
done
clear
View Answer play_arrow
question_answer 75) Aniline reacts with acetaldehyde to form
A)
Schiffs base
done
clear
B)
carbyl amine
done
clear
C)
immine
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 76) The reagent which forms crystalline osazone derivative when reacted with glucose, is
A)
Fehling solution
done
clear
B)
phenyl hydrazine
done
clear
C)
Benedict solution
done
clear
D)
hydroxyl amine
done
clear
View Answer play_arrow
question_answer 77) Chloramine-T is a
A)
disinfectant
done
clear
B)
antiseptic
done
clear
C)
analgesic
done
clear
D)
antipyretic
done
clear
View Answer play_arrow
question_answer 78) Maximum entropy will be in which of the following?
A)
Ice
done
clear
B)
Liquid water
done
clear
C)
Snow
done
clear
D)
Water vapours
done
clear
View Answer play_arrow
question_answer 79) Which of the, following acts as an oxidising as well as reducing agent?
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
C)
\[NaN{{O}_{3}}\]
done
clear
D)
\[NaN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) Maximum number of hydrogen bonds in\[{{H}_{2}}O\]. is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 81) Number of isomers possible for\[{{C}_{4}}{{H}_{8}}O\]is
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 82) The ability of a given substance to assume two or more crystalline structure is called
A)
amorphism
done
clear
B)
isomorphism
done
clear
C)
polymorphism
done
clear
D)
isomerism
done
clear
View Answer play_arrow
question_answer 83) A cricket ball of 0.5 kg is moving with a velocity of 100 m/s. The wavelength associated with its motion is
A)
\[1\text{/}100\,cm\]
done
clear
B)
\[6.6\times {{10}^{-34}}m\]
done
clear
C)
\[1.32\times {{10}^{-35}}m\]
done
clear
D)
\[6.6\times {{10}^{-28}}m\]
done
clear
View Answer play_arrow
question_answer 84) At \[90{}^\circ C,\]pure water has\[{{H}_{3}}{{O}^{+}}\]ion concentration of \[{{10}^{-6}}mol\text{/}L.\] The\[{{K}_{w}}\]at\[90{}^\circ C\] is
A)
\[{{10}^{-6}}\]
done
clear
B)
\[{{10}^{-14}}\]
done
clear
C)
\[{{10}^{-12}}\]
done
clear
D)
\[{{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 85) Aspirin is chemically
A)
methyl benzoate
done
clear
B)
ethyl salicylate
done
clear
C)
acetyl salicylic acid
done
clear
D)
o-hydroxy benzoic acid
done
clear
View Answer play_arrow
question_answer 86) Synthetic polymer which resembles natural rubber, is
A)
neoprene
done
clear
B)
chloroprene
done
clear
C)
glyptal
done
clear
D)
nylon
done
clear
View Answer play_arrow
question_answer 87) The reduction of which of the following compounds would yield secondary amine?
A)
Alkyl nitrile.
done
clear
B)
Carbylamine
done
clear
C)
Primary amine
done
clear
D)
Secondary nitro compound
done
clear
View Answer play_arrow
question_answer 88) Which of the following aldehydes is most reactive?
A)
\[{{C}_{6}}{{H}_{5}}-CHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[HCHO\]
done
clear
D)
All the equally reactive
done
clear
View Answer play_arrow
question_answer 89) Which of the following is dihydric alcohol?
A)
Glycerol
done
clear
B)
Ethylene glycol
done
clear
C)
Carechol
done
clear
D)
Resorcinol
done
clear
View Answer play_arrow
question_answer 90) Ethyl alcohol is heated with cone \[{{H}_{2}}S{{O}_{4}}.\] The product formed is
A)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{\mathbf{||}}{\mathop{C}}\,}}\,-O{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 91) In the first order reaction, the concentration of the reactants is reduced to 25% in one hour. The half-life period of the reaction is
A)
2h
done
clear
B)
4h
done
clear
C)
1/2 h
done
clear
D)
1/4 h
done
clear
View Answer play_arrow
question_answer 92) The movement of solvent molecules through a semipermeable membrane is called
A)
electrolysis
done
clear
B)
electrophoresis
done
clear
C)
osmosis
done
clear
D)
cataphoresis
done
clear
View Answer play_arrow
question_answer 93) Which of the following is a primary halide?
A)
Isopropyl iodide
done
clear
B)
Secondary butyl iodide
done
clear
C)
Tertiay butyl bromide
done
clear
D)
Neohexyl chloride
done
clear
View Answer play_arrow
question_answer 94) \[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\xrightarrow{Cr{{O}_{2}}C{{l}_{2}}}Z\]In the given sequence Z is
A)
benzaldehyde
done
clear
B)
tolueic acid
done
clear
C)
phenyl acetic acid
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 95) In the electrolysis of water, one faraday of electrical energy would evolve
A)
one mole of oxygen
done
clear
B)
one g atom of oxygen
done
clear
C)
8 g of oxygen
done
clear
D)
22.4 L of oxygen
done
clear
View Answer play_arrow
question_answer 96) If the half-life of an isotope X is 10 yr, its decay constant is
A)
6.932\[y{{r}^{-1}}\]
done
clear
B)
0.6932\[y{{r}^{-1}}\]
done
clear
C)
0.06932\[y{{r}^{-1}}\]
done
clear
D)
0.006932\[y{{r}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 97) On strong heating sodium bicarbonate changes into
A)
sodium monoxide
done
clear
B)
sodium hydroxide
done
clear
C)
sodium carbonate
done
clear
D)
sodium peroxide
done
clear
View Answer play_arrow
question_answer 98) Aluminium reacts with caustic soda to form
A)
aluminium hydroxide
done
clear
B)
aluminium oxide
done
clear
C)
sodium meta aluminate
done
clear
D)
sodium tetra aluminate
done
clear
View Answer play_arrow
question_answer 99) Iron is dropped in dil. \[HN{{O}_{3}}\] it gives
A)
ferric nitrate
done
clear
B)
ferric nitrate and\[N{{O}_{2}}\]
done
clear
C)
ferrous nitrate and ammonium nitrate
done
clear
D)
ferrous nitrate and nitric oxide
done
clear
View Answer play_arrow
question_answer 100) The chief impurity present in red bauxite is
A)
\[Si{{O}_{2}}\]
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[NaF\]
done
clear
View Answer play_arrow
question_answer 101) Which one is related to urine concentration in mammals?
A)
Tesrosterone hormone
done
clear
B)
Antidiuretic hormone
done
clear
C)
Oxytocin honnone
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 102) An enzyme that uses viral RNA as template for the synthesis of DNA is
A)
RNA polymerase
done
clear
B)
reverse transcriptase
done
clear
C)
viral nuclease
done
clear
D)
RNA replicase
done
clear
View Answer play_arrow
question_answer 103) The carrier which transfer the electrons in electron transport system
A)
phytochrome
done
clear
B)
cytochrome
done
clear
C)
quantasomes
done
clear
D)
fucoxanthin
done
clear
View Answer play_arrow
question_answer 104) The source of agar-agar is
A)
Chlamydomonas
done
clear
B)
Chlorella.
done
clear
C)
Gelidium
done
clear
D)
Spirogyra
done
clear
View Answer play_arrow
question_answer 105) A term helotism is used for the symbiosis of
A)
alagae and fungi
done
clear
B)
algae and Cycas
done
clear
C)
algae and bacteria
done
clear
D)
Pimis and fungi
done
clear
View Answer play_arrow
question_answer 106) Haploid structure of Funaria is
A)
calyptra
done
clear
B)
protonema
done
clear
C)
apophysis
done
clear
D)
operculum
done
clear
View Answer play_arrow
question_answer 107) In the angiosperm ovule, central cell of the embryo sac, prior to the entry of pollen tube, contains
A)
single haploid nucleus
done
clear
B)
one diploid and one haploid nuclei
done
clear
C)
two haploid polar nuclei
done
clear
D)
one diploid secondary nucleus
done
clear
View Answer play_arrow
question_answer 108) Which of the following is not an influence of auxins?
A)
Apical dominance
done
clear
B)
Parthenocarpy
done
clear
C)
Tropic movements
done
clear
D)
Bolting
done
clear
View Answer play_arrow
question_answer 109) Mycorrhiza helps in
A)
nutrition up taking
done
clear
B)
food manufacturing
done
clear
C)
disease resistance
done
clear
D)
disease prevention
done
clear
View Answer play_arrow
question_answer 110) Amphids are cuticular elevations on ventro tips of Ascaris, These are
A)
tangoreceptors
done
clear
B)
taetoreceptors
done
clear
C)
olfactoreceptors
done
clear
D)
chemoreceptors
done
clear
View Answer play_arrow
question_answer 111) Meiosis in Dryopteris takes-place during
A)
gamete formation
done
clear
B)
spore germination
done
clear
C)
zygote formation
done
clear
D)
spore formation
done
clear
View Answer play_arrow
question_answer 112) Which malaria parasite has longest incubation period?
A)
Plasmodium vivax
done
clear
B)
P. falciparum
done
clear
C)
P. malariae
done
clear
D)
P. ovale
done
clear
View Answer play_arrow
question_answer 113) In which group of the following would you place the plants having vascular tissue and lacking seeds?
A)
Algae
done
clear
B)
Fungi
done
clear
C)
Bryophytes
done
clear
D)
Pteridophytes
done
clear
View Answer play_arrow
question_answer 114) The poisonous fluid present in nematocyst of Hydra is
A)
venom
done
clear
B)
hematin
done
clear
C)
toxin
done
clear
D)
hypnotoxin
done
clear
View Answer play_arrow
question_answer 115) XXY chromosome constitution is represented by
A)
Downs syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Klinefelters syndrome
done
clear
D)
Okazaki syndrome
done
clear
View Answer play_arrow
question_answer 116) The enzyme responsible for the reduction of molecular nitrogen to the level of ammonia in the leguminous root nodule
A)
amminase
done
clear
B)
nitrogenase
done
clear
C)
nitrate reductase
done
clear
D)
nitrite reductase
done
clear
View Answer play_arrow
question_answer 117) Centromere is present at one end, the chromosome is
A)
metacentric
done
clear
B)
excentric
done
clear
C)
telocentric
done
clear
D)
apocentric
done
clear
View Answer play_arrow
question_answer 118) Xenia refers to
A)
effect of pollen on stem
done
clear
B)
effect of pollen on taste of fruit
done
clear
C)
effect of pollen on vascular tissue
done
clear
D)
effect of pollen on endosperm
done
clear
View Answer play_arrow
question_answer 119) Elater mechanism of spore dispersal is exhibited by
A)
Riccia
done
clear
B)
Funaria
done
clear
C)
Liverworts
done
clear
D)
Marchantia
done
clear
View Answer play_arrow
question_answer 120) A chemical fertilizing is produced form
A)
polar bodies
done
clear
B)
middle piece of sperm
done
clear
C)
acrosome
done
clear
D)
mature eggs
done
clear
View Answer play_arrow
question_answer 121) The Indian wild ass is in the category of...... by wild life protection act of government of India
A)
rare species
done
clear
B)
endangered species
done
clear
C)
endemic species
done
clear
D)
vulnerable species
done
clear
View Answer play_arrow
question_answer 122) Which of the following commonly called emergency gland of body?
A)
Thymus
done
clear
B)
Testis
done
clear
C)
Adrenal
done
clear
D)
Pituitary
done
clear
View Answer play_arrow
question_answer 123) Insectivorous plants grow in ...... deficient soil
A)
\[Na\]
done
clear
B)
\[N\]
done
clear
C)
\[C\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 124) Uterine endometrium, epithelial glands and connective tissue are broken in menstrual phase. That is due to
A)
over secretion of FSH
done
clear
B)
lack of estrogen
done
clear
C)
lack of progesterone
done
clear
D)
over production of progesterone
done
clear
View Answer play_arrow
question_answer 125) The organelles, which take part in photo respiration are
A)
chloroplast, mitochondria, nucleus
done
clear
B)
chloroplast, mitochondria, lysosome
done
clear
C)
mitochondria, chloroplast, peroxisome
done
clear
D)
mitochondria, lysosome, peroxisome
done
clear
View Answer play_arrow
question_answer 126) Which of the following is correctly matched pair of a certain plant family and its one example?
A)
Malvaceae-Cotton
done
clear
B)
Leguminosae-Mango or sunflower
done
clear
C)
Cucurbitaceae-Orange
done
clear
D)
Brassicaceae-Wheat
done
clear
View Answer play_arrow
question_answer 127) Which one of the following is a skull bone?
A)
Coracoid
done
clear
B)
Arytaenoid
done
clear
C)
Atlas
done
clear
D)
Pterygoid
done
clear
View Answer play_arrow
question_answer 128) A water fern, which is used as a green manure in rice fields is
A)
Salvinia
done
clear
B)
Mucor
done
clear
C)
Aspergillus
done
clear
D)
Azolla
done
clear
View Answer play_arrow
question_answer 129) In which of the following animal, post anal tail is found?
A)
Earthworm
done
clear
B)
Lower invertebrate
done
clear
C)
Scorpion
done
clear
D)
Snake
done
clear
View Answer play_arrow
question_answer 130) Apogamy is
A)
reproduction of virus
done
clear
B)
failure of fusion of gametes
done
clear
C)
development of bacteria
done
clear
D)
loss of function of reproduction
done
clear
View Answer play_arrow
question_answer 131) Which bacteria are utilized in gobar gas plane?
A)
Methanogens
done
clear
B)
Nitrifying bacteria
done
clear
C)
Ammonifying bacteria
done
clear
D)
Denitrifying bacteria
done
clear
View Answer play_arrow
question_answer 132) In photosynthesis, energy from light reaction to dark reaction is transferred in the form of
A)
ADP
done
clear
B)
ATP
done
clear
C)
RuBP
done
clear
D)
chlorophyll
done
clear
View Answer play_arrow
question_answer 133) ELISA is used to detect viruses where the key reagent is
A)
DNA probe
done
clear
B)
RNAse
done
clear
C)
alkaline phosphatase
done
clear
D)
catalase
done
clear
View Answer play_arrow
question_answer 134) If Henles loop were absent from mammalian nephron, which of the following is to be expected?
A)
The urine will be more concentrated
done
clear
B)
The urine will be more dilute
done
clear
C)
There will be no urine formation
done
clear
D)
There will be hardly any change in the quality and quantity of urine formed
done
clear
View Answer play_arrow
question_answer 135) Which one of the following does not act as a neurotransmitter?
A)
Acetylcholine
done
clear
B)
Epinephrine
done
clear
C)
Norepinephrine
done
clear
D)
Cortisone
done
clear
View Answer play_arrow
question_answer 136) In which of the following stage, the chromosome is single, thin and like long thread?
A)
Leptotene
done
clear
B)
Zygotene
done
clear
C)
Pachytene
done
clear
D)
Diakinesis
done
clear
View Answer play_arrow
question_answer 137) \[{{C}_{4}}\]-plants differ from \[{{C}_{3}}\]-plants in respect to
A)
number of ATP used
done
clear
B)
substrate which accept the\[C{{O}_{2}}\]molecules
done
clear
C)
the final product
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 138) Gonads of Obelia occur in
A)
on blastocyst
done
clear
B)
in hydrula stage
done
clear
C)
radial canals of medusa
done
clear
D)
bases of tentacles of medusa
done
clear
View Answer play_arrow
question_answer 139) The functional xylem of dicot tree is
A)
sap wood
done
clear
B)
hardwood
done
clear
C)
heart wood
done
clear
D)
autumn wood
done
clear
View Answer play_arrow
question_answer 140) Botanical name of sanjeevani is
A)
Selaginella chrysocaulos
done
clear
B)
Selaginella bryopteris
done
clear
C)
Selaginella chrysorhizos
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 141) Elbow joint is
A)
ball and socket
done
clear
B)
hinge joint
done
clear
C)
pivot joint
done
clear
D)
saddle joint
done
clear
View Answer play_arrow
question_answer 142) The point in eye of mammals from which optic nerves and blood vessels leaves the eye ball is
A)
yellow spot
done
clear
B)
blind spot
done
clear
C)
pars optica
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 143) Cerebral hemispheres of rat are connected by
A)
corpus luteum
done
clear
B)
corpus callosum
done
clear
C)
corpus albicans
done
clear
D)
corpus spongiosum
done
clear
View Answer play_arrow
question_answer 144) The nucleolus is the site of formation of
A)
spindle fibres
done
clear
B)
chromosomes
done
clear
C)
ribosomes
done
clear
D)
peroxisomes
done
clear
View Answer play_arrow
question_answer 145) Deposition of uric acid crystals within the synovial joint causes
A)
osteoarthritis
done
clear
B)
rheumatoid arthritis
done
clear
C)
gout
done
clear
D)
paralysis
done
clear
View Answer play_arrow
question_answer 146) X-chromosomes of female in a sex-linked inheritance case can be passed on to
A)
only female progeny
done
clear
B)
only male progeny
done
clear
C)
only in grand daughter
done
clear
D)
male and female progeny
done
clear
View Answer play_arrow
question_answer 147) Leaf in young condition in fern is called
A)
scale leaf
done
clear
B)
sporophyll
done
clear
C)
circulate ptyxis
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 148) What is common in photosynthesis and respiration?
A)
Light energy
done
clear
B)
\[NADP{{H}_{2}}\]
done
clear
C)
Cytochromes
done
clear
D)
\[NAD{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 149) Smell of urine is due to the
A)
urochrome
done
clear
B)
urinode
done
clear
C)
urea
done
clear
D)
melanin
done
clear
View Answer play_arrow
question_answer 150) Sertoli cells are involved in
A)
excretion
done
clear
B)
nutrition of sperms
done
clear
C)
respiration
done
clear
D)
All of these
done
clear
View Answer play_arrow