A) \[Cl-C{{H}_{2}}-C{{H}_{2}}-OH\]
B)
![]()
C)
![]()
D)
![]()
Correct Answer: B
Solution :
Phenols are much more acidic than alcohols due to the stabilisation of phenoxide ion by resonance.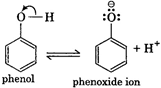 Phenoxide ion is stabilised due to following resonating structures
Phenoxide ion is stabilised due to following resonating structures 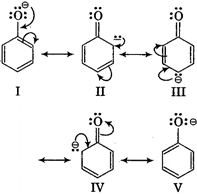 While in alcohols \[\underset{alcohol}{\mathop{R-:\overset{\centerdot \,\,\,\centerdot }{\mathop{O}}\,:-H}}\,\underset{\begin{smallmatrix} not\,stabilised\,due\, \\ to\,absence\,\,of\, \\ resonance \end{smallmatrix}}{\mathop{R-:\overset{\centerdot \,\,\,\centerdot }{\mathop{O}}\,:+{{H}^{+}}}}\,\] ortho-nitrophenol is most acidic because in it \[-N{{O}_{2}}\]i.e., electron attracting group is attached on ortho position which helps in stabilising negative charge on the oxygen of phenoxide ion. Hence, due to this reason acidic character of phenol is increased, while on attachment of\[-C{{H}_{3}}\]group (electron donating group) acidic strength of phenol is decreased in cresol due to destabilisation of phenoxide ion.
While in alcohols \[\underset{alcohol}{\mathop{R-:\overset{\centerdot \,\,\,\centerdot }{\mathop{O}}\,:-H}}\,\underset{\begin{smallmatrix} not\,stabilised\,due\, \\ to\,absence\,\,of\, \\ resonance \end{smallmatrix}}{\mathop{R-:\overset{\centerdot \,\,\,\centerdot }{\mathop{O}}\,:+{{H}^{+}}}}\,\] ortho-nitrophenol is most acidic because in it \[-N{{O}_{2}}\]i.e., electron attracting group is attached on ortho position which helps in stabilising negative charge on the oxygen of phenoxide ion. Hence, due to this reason acidic character of phenol is increased, while on attachment of\[-C{{H}_{3}}\]group (electron donating group) acidic strength of phenol is decreased in cresol due to destabilisation of phenoxide ion.
You need to login to perform this action.
You will be redirected in
3 sec
