A) In adiabatic process, the system exchange heat with surroundings
B) Isothermal process is carried out at constant temperature
C) In isobaric process, change of state is brought with change in pressure
D) In isobaric process, the temperature is kept constant
Correct Answer: B
Solution :
Adiabatic process The system does not exchange heat with the surroundings,\[dq=0\] Isothermal process It is carried out at constant temperature,\[dT=0\]and thus, \[\Delta E=0\] Isobaric process Change of state is brought about at constant pressure, i.e. \[dp=0\] Isochoric process Volume of the system remains constant, i.e. \[dp=0\]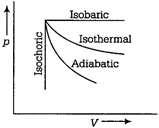
You need to login to perform this action.
You will be redirected in
3 sec
