question_answer 1) Dimensional formula for \[{{\varepsilon }_{0}}\] is
A)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{\text{-1}}}{{\text{L}}^{\text{-2}}}{{\text{A}}^{\text{2}}}{{\text{T}}^{\text{2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{-2}}}{{\text{A}}^{\text{-2}}}{{\text{T}}^{4}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{-1}}{{\text{L}}^{-3}}{{\text{A}}^{\text{2}}}{{\text{T}}^{4}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{3}}{{\text{A}}^{\text{-2}}}{{\text{T}}^{-4}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 2) The velocity v (in cm/s) of a particle is given in terms of time t (in sec) by the relation \[y=at+\frac{b}{t+c};\] the dimensions of a, b and c are
A)
\[a\,\text{=}\,\text{ }\!\![\!\!\text{ }{{\text{L}}^{\text{2}}}\text{ }\!\!]\!\!\text{ ,}\,\text{b}\,\text{=}\,\text{ }\!\![\!\!\text{ T }\!\!]\!\!\text{ ,}\,\text{c}\,\text{=}\,\text{ }\!\![\!\!\text{ L}{{\text{T}}^{\text{2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[a\,\text{=}\,\text{ }\!\![\!\!\text{ L}{{\text{T}}^{\text{2}}}\text{ }\!\!]\!\!\text{ ,}\,\text{b}\,\text{=}\,\text{ }\!\![\!\!\text{ LT }\!\!]\!\!\text{ ,}\,\text{c}\,\text{=}\,\text{ }\!\![\!\!\text{ L }\!\!]\!\!\text{ }\]
done
clear
C)
\[a\,\text{=}\,\text{ }\!\![\!\!\text{ L}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ ,}\,\text{b}\,\text{=}\,\text{ }\!\![\!\!\text{ L }\!\!]\!\!\text{ ,}\,\text{c}\,\text{=}\,\text{ }\!\![\!\!\text{ T }\!\!]\!\!\text{ }\]
done
clear
D)
\[a\,\text{=}\,\text{ }\!\![\!\!\text{ L }\!\!]\!\!\text{ ,}\,\text{b}\,\text{=}\,\text{ }\!\![\!\!\text{ LT }\!\!]\!\!\text{ ,}\,\text{c}\,\text{=}\,\text{ }\!\![\!\!\text{ }{{\text{T}}^{2}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 3) Car A starts initially with the acceleration \[{{a}_{1}},\] after 2 s the car B starts with acceleration \[{{a}_{2}}.\]If in \[{{\text{5}}^{\text{th}}}\text{s}\] both car travels same distance, then the ratio of a \[{{a}_{1}}\] and \[{{a}_{2}}\] will be
A)
5 : 9
done
clear
B)
5 : 7
done
clear
C)
9 : 5
done
clear
D)
9 : 7
done
clear
View Answer play_arrow
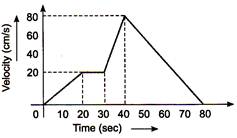
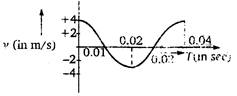
question_answer 4)
The \[\text{v-t}\] graph of a moving object is given in figure. The maximum acceleration is
A)
\[1\text{ }cm/{{s}^{2}}\]
done
clear
B)
\[\text{2 }cm/{{s}^{2}}\]
done
clear
C)
\[\text{3 }cm/{{s}^{2}}\]
done
clear
D)
\[\text{6 }cm/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 5) A 2 kg stone at the end of a string 1 m long is whirled in a vertical circle at a constant speed. The speed of the stone is 4 m/s. The tension in the string will be 52 N, when the stone is
A)
at the top of the circle
done
clear
B)
at the bottom of the circle
done
clear
C)
halfway down
done
clear
D)
None of the above
done
clear
View Answer play_arrow
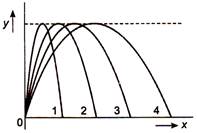
question_answer 6)
Figure shows four paths for a kicked football. Ignoring the effects of air on the flight, rank the paths according to initial horizontal velocity component, highest first
A)
1, 2, 3, 4
done
clear
B)
2, 3, 4,1
done
clear
C)
3, 4, 1, 2
done
clear
D)
4, 3, 2, 1
done
clear
View Answer play_arrow
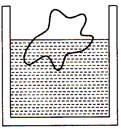
question_answer 7)
A body floats in a liquid contained in a beaker. If the whole system as shown in figure falls freely under gravity, then the upthrust on the body due to liquid is
A)
zero
done
clear
B)
equal to the weight of liquid displaced
done
clear
C)
equal to the weight of the body in air
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 8)
Two carts of masses 200 kg and 300 kg on horizontal rails are pushed apart. Suppose the coefficient of friction between the carts and the rails are same. If the 200 kg cart travels a distance of 36 m and stops, then the distance travelled by the cart weighing 300 kg is
A)
32m
done
clear
B)
24m
done
clear
C)
16m
done
clear
D)
12m
done
clear
View Answer play_arrow
question_answer 9) The force constant of weightless spring is 16 N/m. A body of mass 1.0 kg suspended from it is pulled down through 5 cm and then released. The maximum kinetic energy of the system (spring +body) will be
A)
\[\text{2 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
B)
\[\text{4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
C)
\[\text{8 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
D)
\[\text{16 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
View Answer play_arrow
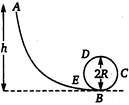
question_answer 10)
A frictionless track A B C D E ends in a circular loop of radius R. A body slides down the track from point A which is at a height h = S cm. Maximum value of R for the body to successfully complete the loop is
A)
\[\text{5}\,\text{cm}\]
done
clear
B)
\[\frac{15}{4}\,\text{cm}\]
done
clear
C)
\[\frac{10}{3}\,\text{cm}\]
done
clear
D)
\[\text{2}\,\text{cm}\]
done
clear
View Answer play_arrow
question_answer 11) A constant torque acting on a uniform circular wheel changes its angular momentum from \[{{\text{A}}_{\text{0}}}\] to \[\text{4}{{\text{A}}_{\text{0}}}\] in 4 s. The magnitude of this torque is
A)
\[\frac{3{{A}_{0}}}{4}\]
done
clear
B)
\[{{A}_{0}}\]
done
clear
C)
\[4{{A}_{0}}\]
done
clear
D)
\[121{{A}_{0}}\]
done
clear
View Answer play_arrow
question_answer 12) The rotational kinetic energy of a body is KE and its moment of inertia is \[I\] The angular momentum is
A)
\[KEI\]
done
clear
B)
\[2\sqrt{KEI}\]
done
clear
C)
\[\sqrt{2KEI}\]
done
clear
D)
\[KE/I\]
done
clear
View Answer play_arrow
question_answer 13) A planet is moving around the sun in an elliptic orbit. Its speed
A)
is the same at all points of the orbit
done
clear
B)
is maximum when it is farthest from the sun
done
clear
C)
is maximum when it is nearest to the sun
done
clear
D)
is maximum at the two points in which the orbit is intersected by the line which passes through the focus of the orbit and which is perpendicular to its major axis
done
clear
View Answer play_arrow
question_answer 14) Rohini satellite at a height of 500 km and INSAT-B at a height of 3600 km from surface of earth, then relation between their orbital velocity \[({{v}_{R,}}{{v}_{I}})\] is
A)
\[({{v}_{R}}>{{v}_{I}})\]
done
clear
B)
\[({{v}_{R}}<{{v}_{I}})\]
done
clear
C)
\[{{v}_{R}}={{v}_{I}})\]
done
clear
D)
No relation
done
clear
View Answer play_arrow
question_answer 15) A wire of cross-sectional area 3 mm2 is first stretched between two fixed points at a temperature of \[20{}^\circ C\]. Determine the tension when the temperature falls to \[10{}^\circ C\]. Coefficient of linear expansion \[a={{10}^{5}}{{\,}^{\text{o}}}{{\text{C}}^{-1}}\]and
A)
20 N
done
clear
B)
30 N
done
clear
C)
60 N
done
clear
D)
120 N
done
clear
View Answer play_arrow
question_answer 16) If the length of a wire is reduced to half, then it can hold the................. load
A)
half
done
clear
B)
same
done
clear
C)
double
done
clear
D)
one-fourth
done
clear
View Answer play_arrow
question_answer 17) If two soap bubbles of different radii are in communication with each other
A)
Air flows from larger bubble into the smaller one
done
clear
B)
The size of the bubbles remains the same
done
clear
C)
Air flows from the smaller bubble into the larger one and the larger bubble grows at the expense of the smaller one
done
clear
D)
The air flows from the larger
done
clear
View Answer play_arrow
question_answer 18) Two capillary tubes of same diameter are put vertically one each in two liquids whose relative densities are 0.8 and 0.6 and surface tensions are 60 and 50 dyne cm respectively ratio of heights of liquids in the two tubes is
A)
10/9
done
clear
B)
3/10
done
clear
C)
10/3
done
clear
D)
9/10
done
clear
View Answer play_arrow
question_answer 19) At which temperature the velocity of \[{{O}_{2}}\] molecules will be equal to the velocity of \[{{N}_{2}}\] molecules at \[O{{\,}^{\text{o}}}\text{C}\] ?
A)
\[40{}^\circ C\]
done
clear
B)
\[93{}^\circ C\]
done
clear
C)
\[39{}^\circ C\]
done
clear
D)
Cannot be calculated
done
clear
View Answer play_arrow
question_answer 20) If the volume of the gas containing m number of molecules is V, then the pressure will decrease due to force of intermolecular attraction in the proporation.
A)
\[n/V\]
done
clear
B)
\[n/{{V}^{2}}\]
done
clear
C)
\[~{{(n/V)}^{2}}\]
done
clear
D)
\[1/{{V}^{2}}\]
done
clear
View Answer play_arrow
question_answer 21) Work done in converting one gram of ice at WC into steam at \[100{}^\circ C\] is
A)
3045 J
done
clear
B)
6056 J
done
clear
C)
721 J
done
clear
D)
616 J
done
clear
View Answer play_arrow
question_answer 22) A Carnot engine working between 300 K and 600 K has a work output of 800 J per cycle. What is the amount of heat energy supplied to the engine from source per cycle ?
A)
1200 J/cycle
done
clear
B)
1600 J/cycle
done
clear
C)
1400 J/cycle
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 23) Two large closely spaced concentric spheres (both are black body radiators) are maintained at temperature of 200 K and 300 K respectively. The space between them is evacuated. The net rate of energy transfer between the two spheres will be (\[\sigma \]\[=5.672\times {{10}^{8}}MKS\] unit)
A)
368.68W/m2
done
clear
B)
3686.8 W/m2
done
clear
C)
36.868 W/m2
done
clear
D)
36868 W/m2
done
clear
View Answer play_arrow
question_answer 24) Two identical vessels made up of same material are filled with same amount of ice. If in the vessels the ice melts in time \[{{t}_{1}}\] and \[{{t}_{2}}\] respectively, then the ratio of their thermal conductivities will be
A)
\[{{t}_{2}}:{{t}_{1}}\]
done
clear
B)
\[{{t}_{1}}:{{t}_{2}}\]
done
clear
C)
\[t_{2}^{2}:t_{1}^{2}\]
done
clear
D)
\[t_{1}^{2}:t_{2}^{2}\]
done
clear
View Answer play_arrow
question_answer 25)
The velocity-time diagram of harmonic oscillator is shown in the adjoining figure. The frequency of oscillation is
A)
\[\text{25}\,\text{Hz}\]
done
clear
B)
\[\text{50}\,\text{Hz}\]
done
clear
C)
\[12.25\,\text{Hz}\]
done
clear
D)
\[33\,\text{Hz}\]
done
clear
View Answer play_arrow
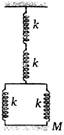
question_answer 26)
The frequency of oscillation of system shown in the figure will be
A)
\[\frac{1}{2\pi }\sqrt{\frac{k}{M}}\]
done
clear
B)
\[\frac{1}{2\pi }\sqrt{\frac{2k}{5M}}\]
done
clear
C)
\[\frac{1}{2\pi }\sqrt{\frac{k}{5M}}\]
done
clear
D)
\[\frac{1}{2\pi }\sqrt{\frac{2k}{M}}\]
done
clear
View Answer play_arrow
question_answer 27)
On a smooth inclined plane, a body of mass M b attached between two springs. The other ends of the springs are fixed to firm supports. If each spring has force constant k, the period of oscillation of the body (assuming the springs as massless) is
A)
\[2\pi {{\left( \frac{M}{2k} \right)}^{1/2}}\]
done
clear
B)
\[2\pi {{\left( \frac{2M}{k} \right)}^{1/2}}\]
done
clear
C)
\[2\pi \frac{Mg\,\sin \,\text{ }\!\!\theta\!\!\text{ }}{2k}\]
done
clear
D)
\[2\pi {{\left( \frac{2Mg}{k} \right)}^{1/2}}\]
done
clear
View Answer play_arrow
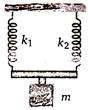
question_answer 28)
in the arrangement shown in the figure, for vertical oscillations of the mass m, the period is
A)
\[T=2\pi \sqrt{\frac{m}{{{k}_{1}}-{{k}_{2}}}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{m}}\]
done
clear
C)
\[T=2\pi \sqrt{\frac{m({{k}_{1}}+{{k}_{2}})}{{{k}_{1}}{{k}_{2}}}}\]
done
clear
D)
\[T=2\pi \sqrt{\frac{mg}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
View Answer play_arrow
question_answer 29) A string fixed at both the ends is vibrating in two segments. The wavelength of the corresponding time is
A)
\[l/4\]
done
clear
B)
\[l/2\]
done
clear
C)
\[l\]
done
clear
D)
\[2l\]
done
clear
View Answer play_arrow
question_answer 30) A sitar wire vibrates with frequency 330 vib/s. If its length is increased three times and tension is increased four times, then the frequency of the wire will be
A)
330 Hz
done
clear
B)
220 Hz
done
clear
C)
110 Hz
done
clear
D)
440 Hz
done
clear
View Answer play_arrow
question_answer 31) A wire of 1m length and \[9\times {{10}^{3}}g/{{m}^{3}}\] density is clamped between two points. If its extension on loading is 0.36 mm, then frequency of small transverse vibration is (Young's modulus \[=9\times {{10}^{10}}N/{{m}^{2}}\])
A)
30 Hz
done
clear
B)
40 Hz
done
clear
C)
50 Hz
done
clear
D)
60 Hz
done
clear
View Answer play_arrow
question_answer 32) The length of a sonometer wire AB is 110 cm. Where should the two bridges be placed from A to divide the wire in 3 segments whose fundamental frequencies are in the ratio of =1:2:3
A)
30 cm and 60 cm
done
clear
B)
40 cm and 80 cm
done
clear
C)
60 cm and 90 cm
done
clear
D)
30 cm and 90 cm
done
clear
View Answer play_arrow
question_answer 33) If the amplitude ratio of two sources producing interference is 3 : 5, the ratio of intensities at maxima and minima is
A)
25 : 16
done
clear
B)
5 : 3
done
clear
C)
16 : 1
done
clear
D)
25 : 9
done
clear
View Answer play_arrow
question_answer 34) In two separate set-ups of the Young's double slit experiment fringes of equal width are observed when lights of wavelengths in the ratio 1:2 are- used. If the ratio of the slit separation in the two cases is 2:1 the ratio of the distance between the plane of the slits and the screen in the two set-ups is
A)
4:1
done
clear
B)
1:1
done
clear
C)
1:4
done
clear
D)
2:1
done
clear
View Answer play_arrow
question_answer 35) Radius of curvature of concave mirror is 40 cm and the size of image is twice as that of object, then the object distance is
A)
60 cm
done
clear
B)
20 cm
done
clear
C)
40cm
done
clear
D)
30cm
done
clear
View Answer play_arrow
question_answer 36) If an object is placed 10 cm in front of a concave mirror of focal length 20 cm, the image will be
A)
diminished, upright, virtual
done
clear
B)
enlarged, upright, virtual
done
clear
C)
diminished, inverted, real
done
clear
D)
enlarged, upright, real
done
clear
View Answer play_arrow
question_answer 37) It is desired to photograph the image of an object placed at a distance of 3 m from the plane mirror. The camera which is at a distance of 4.5 m from the mirror should be focused for a distance of
A)
3 m
done
clear
B)
4.5 m
done
clear
C)
6 m
done
clear
D)
7.5 m
done
clear
View Answer play_arrow
question_answer 38) A white screen illuminated by green and red light appears to be
A)
green
done
clear
B)
red
done
clear
C)
yellow
done
clear
D)
white
done
clear
View Answer play_arrow
question_answer 39) The critical angle of light passing from glass to air is minimum for
A)
red light
done
clear
B)
yellow light
done
clear
C)
green light
done
clear
D)
violet light
done
clear
View Answer play_arrow
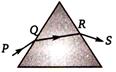
question_answer 40)
A ray of light is incident on a equilateral prism kept on a horizontal table (as shown). Which of the following statement is wrong for the angle of minimum deviation ?
A)
P Q should be parallel to the base
done
clear
B)
Q R should be parallel to the base
done
clear
C)
RS should be parallel to the base
done
clear
D)
Any one of PQ and RS should be horizontal
done
clear
View Answer play_arrow
question_answer 41) A person cannot see objects clearly beyond 50 cm. They power of the lens to correct the vision is
A)
-5.0D
done
clear
B)
-0.5D
done
clear
C)
+2D
done
clear
D)
-2D
done
clear
View Answer play_arrow
question_answer 42) The focal length of objective and eye lens of microscope are 1.6 cm and 2.5 cm respectively. The distance between the two lenses is 21.7 cm. If the final image is formed at infinity, then the distance between the object lens and object is
A)
1.80 cm
done
clear
B)
1.70 cm
done
clear
C)
1.65 cm
done
clear
D)
1.75 cm
done
clear
View Answer play_arrow
question_answer 43) The minimum magnifying power of a telescope is m. If the focal length of its eye lens is halved, the magnifying power will become
A)
m/2
done
clear
B)
2m
done
clear
C)
3m
done
clear
D)
4m
done
clear
View Answer play_arrow
question_answer 44) The rest energy of an electron is 0.511 Me V. The electron is accelerated from rest to a velocity 0.5c. The change in its energy will be
A)
0.026 MeV
done
clear
B)
0.051 MeV
done
clear
C)
0.079 MeV
done
clear
D)
0.105 MeV
done
clear
View Answer play_arrow
question_answer 45) An electron beam has a kinetic energy equal to 100 eV. Find its wavelength associated with a beam, if mass of electron \[=9.1\times {{10}^{-31}}\]kg and 1 eV\[=1.6\times {{10}^{-19}}\]J/eV (Planck's constant \[=6.6\times {{10}^{-34}}\text{J-s})\]
A)
\[6.3\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[3.8\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[24.6\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.12\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 46) The energy of a photon of light of wavelength 450 nm is
A)
\[4.4\times {{10}^{-19}}\text{J}\]
done
clear
B)
\[2.5\times {{10}^{-19}}\text{J}\]
done
clear
C)
\[1.25\times {{10}^{-17}}\text{J}\]
done
clear
D)
\[2.5\times {{10}^{-17}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 47) The wavelength of the most energetic X-ray emitted when a metal target is bombarded by 100 ke V electrons is approximately.
A)
\[12\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.31\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.124\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 48) For production of characteristic \[{{K}_{\text{ }\!\!\beta\!\!\text{ }}}\] X-rays, the electron transition is
A)
\[n=2\,to\,n=1\]
done
clear
B)
\[n=3\,to\,n=2\]
done
clear
C)
\[n=3\,to\,n=1\]
done
clear
D)
\[n=4\,to\,n=2\]
done
clear
View Answer play_arrow
question_answer 49) An X-ray tube operates on 30 kV. What is the minimum wave length emitted ? \[c=3\times {{10}^{8}}\text{m}{{\text{s}}^{\text{-1}}}\text{,}\]\[e=1.6\times {{10}^{-19}}C)\] \[e=1.6\times {{10}^{-19}}C)\]
A)
\[0.133\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.4\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1.2\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[6.6\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 50) Atomic hydrogen is excited to the \[{{\text{n}}^{\text{th}}}\] energy level. The maximum number of spectral lines which it can emit while returning to the ground state is
A)
\[\frac{1}{2}n(n-1)\]
done
clear
B)
\[\frac{1}{2}n(n+1)\]
done
clear
C)
\[n(n-1)\]
done
clear
D)
\[n(n+1)\]
done
clear
View Answer play_arrow
question_answer 51) \[a\]-particles of energy 400 ke V are bombarded on nucleus of \[_{\text{82}}\text{Pb}\text{.}\] In scattering of \[a\]-particles, its minimum distance form nucleus will be
A)
0.59 nm
done
clear
B)
\[0.59\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
5.9pm
done
clear
D)
0.59pm
done
clear
View Answer play_arrow
question_answer 52) If half-life of a radioactive atom is 2.3 days, then its decay constant would be
A)
0.1
done
clear
B)
0.2
done
clear
C)
0.3
done
clear
D)
2.3
done
clear
View Answer play_arrow
question_answer 53) In a good conductor the energy gap between the conduction band and the valence band is
A)
infinite
done
clear
B)
wide
done
clear
C)
narrow
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 54) A semiconductor has an electron concentration density of\[8\times {{10}^{14}}\text{/c}{{\text{m}}^{\text{3}}}\] and that of holes is \[5\times {{10}^{12}}\text{/c}{{\text{m}}^{\text{3}}}\text{.}\] The semiconductor is
A)
n-type
done
clear
B)
p-type
done
clear
C)
intrinsic
done
clear
D)
p-n-p type
done
clear
View Answer play_arrow
question_answer 55) The current relationship between two current gains a and \[\text{ }\!\!\beta\!\!\text{ }\] in a transistor is
A)
\[\text{ }\!\!\beta\!\!\text{ }\,\text{=}\,\frac{a}{1+a}\]
done
clear
B)
\[\text{ }\!\!\beta\!\!\text{ }\,\text{=}\,\frac{a+1}{a}\]
done
clear
C)
\[a\,\text{=}\,\frac{\text{ }\!\!\beta\!\!\text{ }}{a+\text{ }\!\!\beta\!\!\text{ }}\]
done
clear
D)
\[\,\text{ }\!\!\beta\!\!\text{ =}\,\frac{\text{ }\!\!\beta\!\!\text{ +1}}{\text{ }\!\!\beta\!\!\text{ }}\]
done
clear
View Answer play_arrow
question_answer 56) Two bar magnets of the same mass, length and breadth but magnetic moments M and 2 M respectively, when placed in same position, time period is 3 s. What will be the time period when they are placed in different position?
A)
\[\sqrt{2}\,s\]
done
clear
B)
\[3\sqrt{3}\,s\]
done
clear
C)
3 s
done
clear
D)
6 s
done
clear
View Answer play_arrow
question_answer 57) Vibration magnetometer works on the principle of
A)
torque acting on the bar magnet
done
clear
B)
force acting on the bar magnet
done
clear
C)
Both the force and the torque acting on the bar magnet
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 58) The time period of a freely suspended magnet is 2 s. If it is broken in length into two equal parts and one part is suspended in the same way, then its time period will be
A)
4s
done
clear
B)
2s
done
clear
C)
\[\sqrt{2}\,s\]
done
clear
D)
1 s
done
clear
View Answer play_arrow
question_answer 59) Which of the following charge is not possible?
A)
\[1.6\times {{10}^{-19}}C\]
done
clear
B)
\[4.8\times {{10}^{-19}}C\]
done
clear
C)
\[8\times {{10}^{-19}}C\]
done
clear
D)
\[6\times {{10}^{-19}}C\]
done
clear
View Answer play_arrow
question_answer 60) If dielectric constant of water is 81, then its permittivity is
A)
\[7.2\times {{10}^{10}}\frac{{{\text{C}}^{\text{2}}}}{\text{N-}{{\text{m}}^{\text{2}}}}\]
done
clear
B)
\[8.86\times {{10}^{-9}}\frac{{{\text{C}}^{\text{2}}}}{\text{N-}{{\text{m}}^{\text{2}}}}\]
done
clear
C)
\[1.02\times {{10}^{-12}}\frac{{{\text{C}}^{\text{2}}}}{\text{N-}{{\text{m}}^{\text{2}}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 61) An electron of 100 eV energy is projected towards large negatively charged plate having charge density \[-2\times 10{{-}^{6}}C/{{m}^{2}}.\] Then, what must be the distance of electron, so it does not strike the plate?
A)
0.44mm
done
clear
B)
0.44cm
done
clear
C)
044m
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 62) Two opposite and equal charges of \[4\times {{10}^{-8}}C\] when placed \[2\times {{10}^{-2}}cm\] away form a dipole. If this dipole is placed in an external electric field \[4\times {{10}^{8}}N/C,\] the value of maximum torque and the work done in rotating it through \[180{}^\circ C\] will be
A)
\[64\times {{10}^{-4}}Nm\text{ }and\text{ }64\times {{10}^{-4}}J\]
done
clear
B)
\[32\times {{10}^{-4}}Nm\text{ }and\text{ }32\times {{10}^{-4}}J\]
done
clear
C)
\[64\times {{10}^{-4}}Nm\text{ }and\text{ }32\times {{10}^{-4}}J\]
done
clear
D)
\[32\times {{10}^{-4}}Nm\text{ }and\text{ }64\times {{10}^{-4}}J\]
done
clear
View Answer play_arrow
question_answer 63) Water is a good solvent because its molecules are
A)
neutral
done
clear
B)
polar
done
clear
C)
non-polar
done
clear
D)
anode
done
clear
View Answer play_arrow
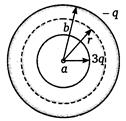
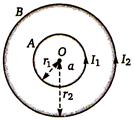
question_answer 64)
In Figure, \[a<r<b\] then intensity at r distance from centre is
A)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left[ \frac{3q}{{{r}^{2}}}-\frac{q}{{{(b-r)}^{2}}} \right]\]
done
clear
B)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left[ \frac{-q}{{{r}^{2}}} \right]\]
done
clear
C)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left( \frac{3q}{{{r}^{2}}} \right)\]
done
clear
D)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\frac{3q}{{{a}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 65) An electron falls through 4 cm in uniform field f \[5\times {{10}^{4}}\text{N/C,}\] then time required must be
A)
\[2.99\times {{10}^{-7}}\text{s}\]
done
clear
B)
\[2.99\times {{10}^{-8}}\text{s}\]
done
clear
C)
\[2.99\times {{10}^{-9}}\text{s}\]
done
clear
D)
\[2.99\times {{10}^{-10}}\text{s}\]
done
clear
View Answer play_arrow
question_answer 66) A capacitor of \[6\mu F\] capacitance is charged up to 100 V. It is touched and then removed with another uncharged capacitor of \[14\mu F,\] then ratio of charges on both and potential at \[6\mu F\] capacitor is
A)
6/14 and 50V
done
clear
B)
3/7 and 30V
done
clear
C)
7/3 and 30V
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 67) Capacity of parallel plate capacitor is \[10\mu F,\] when gap between plates is 8 cm, then what will be its capacity if gap is reduced to 4 cm?
A)
\[10\text{ }\mu F\]
done
clear
B)
\[40\text{ }\mu F\]
done
clear
C)
\[20\text{ }\mu F\]
done
clear
D)
\[30\text{ }\mu F\]
done
clear
View Answer play_arrow
question_answer 68) Capacitor of 12 pF capacitance is connected to 50V battery then electrostatic potential energy will be
A)
\[1.5\times {{10}^{-8}}\text{J}\]
done
clear
B)
\[2.5\times {{10}^{-7}}\text{J}\]
done
clear
C)
\[3.5\times {{10}^{-5}}\text{J}\]
done
clear
D)
\[4.5\times {{10}^{-2}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 69) Two sphere of radii 4 cm and 6 cm are given \[80\text{ }\mu C\] and \[40\text{ }\mu C\]charges and both are joined by wire, then charge will flow.
A)
\[32\mu C\] from A to B
done
clear
B)
\[24\text{ }\mu C\] from A to B
done
clear
C)
\[32\text{ }\mu C\] from B to A
done
clear
D)
None of the above
done
clear
View Answer play_arrow
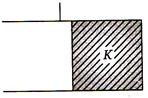
question_answer 70)
If initial capacitance of capacitor is C, then final capacity of capacitor will be
A)
\[KC\]
done
clear
B)
\[(K+1)C\]
done
clear
C)
\[(K+1)C\text{/2}\]
done
clear
D)
\[(K-1)C\]
done
clear
View Answer play_arrow
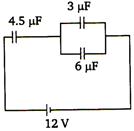
question_answer 71)
Potential difference across capacitor of 4.5 µF capacitance is
A)
8/3 V
done
clear
B)
4V
done
clear
C)
6V
done
clear
D)
8V
done
clear
View Answer play_arrow
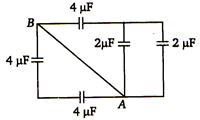
question_answer 72)
Equivalent capacitance between A and B for circuit shown in figure.
A)
\[3\mu F\]
done
clear
B)
\[2\text{ }\mu F\]
done
clear
C)
\[4\text{ }\mu F\]
done
clear
D)
8 µF
done
clear
View Answer play_arrow
question_answer 73) Lengths of two wires are 50 cm, 100 cm respectively and their diameters are 1 mm, 2 mm then ratio between their specific resistances is
A)
1:2
done
clear
B)
1:1
done
clear
C)
2 :1
done
clear
D)
1:4
done
clear
View Answer play_arrow
question_answer 74) A torch battery consisting of two cells of 1.45 V and an internal resistance 0.15\[\Omega \] each cell sending currents through the filament of the lamps having resistance 1.5 \[\Omega .\] The value of current will be.
A)
16.11 A
done
clear
B)
1.611 A
done
clear
C)
0.1611 A
done
clear
D)
2.6 A
done
clear
View Answer play_arrow
question_answer 75) Two wires of same material and same lengths having cross-sections 3:1. If resistance of thicker wire is \[10\,\Omega \] and both are joined in series then equivalent resistance is
A)
\[10\,\Omega \]
done
clear
B)
\[20\,\Omega \]
done
clear
C)
\[30\,\Omega \]
done
clear
D)
\[40\,\Omega \]
done
clear
View Answer play_arrow
question_answer 76) In the measurement of a resistance by Wheatstone bridge the known and the unknown resistances are interchanged to neutralise
A)
end error
done
clear
B)
index error
done
clear
C)
error due to thermoelectric effect
done
clear
D)
random error
done
clear
View Answer play_arrow
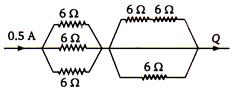
question_answer 77)
Equivalent resistance between A and B of circuit shown in figure.
A)
\[25\,\Omega \]
done
clear
B)
\[35\,\Omega \]
done
clear
C)
\[10\,\Omega \]
done
clear
D)
\[5\,\Omega \]
done
clear
View Answer play_arrow
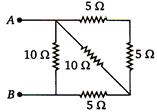
question_answer 78)
Resistances of \[6\,\Omega \] each are connected in the manner shown in adjoining figure. With the current 0.5 A as shown in figure, the potential difference \[{{\text{V}}_{\text{P}}}\text{-}{{\text{V}}_{\text{Q}}}\] is
A)
3.5V
done
clear
B)
6.0V
done
clear
C)
3.0V
done
clear
D)
7.2V
done
clear
View Answer play_arrow
question_answer 79) Which of the following are true when the cells are connected in series ?
A)
Current capacity decreases
done
clear
B)
Current capacity increases
done
clear
C)
The emf decrease
done
clear
D)
The emf increases
done
clear
View Answer play_arrow
question_answer 80) 34 kcal heat is liberated when \[1\text{ }g\text{ }{{H}_{1}}(ECE=1.044\times {{10}^{-8}})\] is converted into water. Minimum voltage required for it is
A)
0.75 V
done
clear
B)
1.5 V
done
clear
C)
3.0V
done
clear
D)
4.5V
done
clear
View Answer play_arrow
question_answer 81) Emf of lead accumulator after complete charging is
A)
2.0V
done
clear
B)
1.8V
done
clear
C)
1.5V
done
clear
D)
2.2V
done
clear
View Answer play_arrow
question_answer 82) ECE is equal to that mass of substance which is deposited on electrode in half sec by current
A)
5 A
done
clear
B)
6 A
done
clear
C)
8A
done
clear
D)
2A
done
clear
View Answer play_arrow
question_answer 83) Ratio of resistance of two bulbs 40 W and 60 W connected across 220 V source is
A)
3:2
done
clear
B)
3:8
done
clear
C)
4:3
done
clear
D)
9:4
done
clear
View Answer play_arrow
question_answer 84) If current is passed through Sb-Bi thermocouple then
A)
heat is produced at junction where current is from Sb the Bi
done
clear
B)
heat is absorbed at junction where current is from Sb to Bi
done
clear
C)
Both junction will become hot
done
clear
D)
Both junction will become cold
done
clear
View Answer play_arrow
question_answer 85) 100 W-220 V bulb is connected to 110 V source. then power consumed is
A)
25 W
done
clear
B)
50 W
done
clear
C)
100 W
done
clear
D)
200 W
done
clear
View Answer play_arrow
question_answer 86) Thermo emf of a couple is \[3\mu \text{ }V{{/}^{0}}C\] if temperature of cold junction is 20'C and thermo emf is increased to 0.3 mV the r. temperature of hot junction
A)
\[80{}^\circ C\]
done
clear
B)
\[100{}^\circ C\]
done
clear
C)
\[120{}^\circ C\]
done
clear
D)
\[140{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 87) A coil has 25 turns and 10 cm radius. If it carries a 4 A current, then magnetic field at its centre is
A)
\[6.28\times {{10}^{-5}}\text{T}\]
done
clear
B)
\[6.28\times {{10}^{-3}}\text{T}\]
done
clear
C)
\[6.28\times {{10}^{-4}}\text{T}\]
done
clear
D)
\[6.28\times {{10}^{-2}}\text{T}\]
done
clear
View Answer play_arrow
question_answer 88)
A and B are two concentric circular conductors of centre 0 and carrying currents \[{{\text{I}}_{\text{1}}}\]and \[{{\text{I}}_{2}}\] as shown in the figure. The ratio of their radii is 1: 2 and ratio of the flux densities at 0 due to A and B is 1 : 3.The value of \[{{\text{I}}_{1}}\text{/}{{\text{I}}_{2}}\]will be
A)
1/6
done
clear
B)
¼
done
clear
C)
1/2
done
clear
D)
1/3
done
clear
View Answer play_arrow
question_answer 89) A power line carrying 10 A current from east to west in earth's magnetic field of 10-T, then force acting on its 1 m length is
A)
zero
done
clear
B)
\[{{10}^{-2}}N\]
done
clear
C)
\[{{10}^{-3}}N\]
done
clear
D)
\[{{10}^{-4}}N\]
done
clear
View Answer play_arrow
question_answer 90) If an electron moving with the kinetic energy of \[6\times {{10}^{-16}}J\] enters perpendicularly into the magnetic field of intensity \[6\times {{10}^{-3}}Wb/\text{ }{{m}^{2}},\] then the radius of the path described by it will be
A)
3.42cm
done
clear
B)
4.23cm
done
clear
C)
5.17cm
done
clear
D)
7.7cm
done
clear
View Answer play_arrow
question_answer 91) A conducting circular loop of radius r carries a constant current \[\text{I}\text{.}\] It is placed in a uniform magnetic field, such that B is perpendicular to the plane of the loop. The magnetic force acting on the loop is given by
A)
\[IrB\]
done
clear
B)
\[2\pi IrB\]
done
clear
C)
zero
done
clear
D)
\[\pi IrB\]
done
clear
View Answer play_arrow
question_answer 92) Emf of generator is 6V and internal resistance is 0.5\[k\,\Omega .\] If internal resistance of voltmeter is 2.5 \[k\,\Omega .\] then reading of voltmeter must be
A)
10-3V
done
clear
B)
1V
done
clear
C)
5V
done
clear
D)
10 V
done
clear
View Answer play_arrow
question_answer 93) In a tangent galvanometer a current of 0.1 A produces a deflection of \[30{}^\circ C\]. The current required to produce a deflection of \[60{}^\circ \] will be
A)
0.6 A
done
clear
B)
0.5 A
done
clear
C)
0.4 A
done
clear
D)
0.3 A
done
clear
View Answer play_arrow
question_answer 94) If magnetic flux linked with a coil is given by \[\phi =5{{t}^{2}}+3t+16.\] Then, induced emf in 4th sec is
A)
10 V
done
clear
B)
-10 V
done
clear
C)
-43V
done
clear
D)
-33V
done
clear
View Answer play_arrow
question_answer 95) Self inductance of coil is L if it is covered with a medium of permeability u, then self inductance becomes
A)
L
done
clear
B)
\[\mu \,\text{L}\]
done
clear
C)
\[\mu {{\,}^{\text{2}}}\text{L}\]
done
clear
D)
\[\,\text{L/}\mu \]
done
clear
View Answer play_arrow
question_answer 96) A copper rod of length; is rotated about one end perpendicular to the uniform magnetic field B with the constant angular velocity\[\omega .\]The induced emf between the two ends is
A)
\[B\omega {{l}^{2}}\text{/2}\]
done
clear
B)
\[B\omega {{l}^{2}}\]
done
clear
C)
\[2B\omega {{l}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 97) The reactance of a coil when used in the domestic AC power supply (220 V, 50 cycles/s) is 50 Q. The inductance of the coil is nearly
A)
2.2 H
done
clear
B)
0.22 H
done
clear
C)
1.6 H
done
clear
D)
0.16 H
done
clear
View Answer play_arrow
question_answer 98) In L-C-R circuit \[{{V}_{L}}={{V}_{C}}={{V}_{R}}=\] 10 V if C is short circuited then voltage across L will be
A)
\[\text{10/}\sqrt{\text{2}}\,\text{V}\]
done
clear
B)
\[20\sqrt{\text{2}}\,\text{V}\]
done
clear
C)
\[10\sqrt{\text{2}}\]
done
clear
D)
\[10\,\text{V}\]
done
clear
View Answer play_arrow
question_answer 99) A coil of resistance R and inductance L is connected to a battery of emf E volts. The final current in the coil is
A)
\[\frac{E}{R}\]
done
clear
B)
\[\frac{E}{L}\]
done
clear
C)
\[\sqrt{\left( \frac{E}{{{R}^{2}}+{{L}^{2}}} \right)}\]
done
clear
D)
\[\sqrt{\left( \frac{EL}{{{R}^{2}}+{{L}^{2}}} \right)}\]
done
clear
View Answer play_arrow
question_answer 100) Which quantity is increased in step down transformer ?
A)
Current
done
clear
B)
Voltage
done
clear
C)
Power
done
clear
D)
Frequency
done
clear
View Answer play_arrow
question_answer 101) For an electron impossible combination of quantum number is
A)
\[n=3,l=2,{{m}_{l}}=-2,{{m}_{s}}=+\frac{1}{2}\]
done
clear
B)
\[n=3,l=2,{{m}_{l}}=-3,{{m}_{s}}=+\frac{1}{2}\]
done
clear
C)
\[n=4,l=0,{{m}_{l}}=-3,{{m}_{s}}=-\frac{1}{2}\]
done
clear
D)
\[n=5,l=3,{{m}_{l}}=0,{{m}_{s}}=-\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 102) The energy of an electron in nth orbit of hydrogen atom is
A)
\[\frac{13.6}{{{n}^{4}}}eV\]
done
clear
B)
\[\frac{13.6}{{{n}^{3}}}eV\]
done
clear
C)
\[\frac{13.6}{{{n}^{2}}}eV\]
done
clear
D)
\[\frac{13.6}{n}eV\]
done
clear
View Answer play_arrow
question_answer 103) Which of the following is not isoelectronic?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[{{O}^{2-}}\]
done
clear
D)
\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 104) The number of unpaired electrons is maximum in (Atomic no. \[Ti=22,V=23,Cr=24,Fe=26)\])
A)
\[Fe\]
done
clear
B)
\[Cr\]
done
clear
C)
\[Ti\]
done
clear
D)
\[V\]
done
clear
View Answer play_arrow
question_answer 105) On moving from left to right in the second period along the periodic table the gram atomic volume of the element
A)
increases with constant velocity
done
clear
B)
remains unchanged
done
clear
C)
first increases and then decreases
done
clear
D)
decreases
done
clear
View Answer play_arrow
question_answer 106) Which of the following is the correct order of the size of iodine species?
A)
\[I>{{I}^{-}}>{{I}^{+}}\]
done
clear
B)
\[{{I}^{-}}>I>{{I}^{+}}\]
done
clear
C)
\[{{I}^{+}}>I>{{I}^{-}}\]
done
clear
D)
\[I>{{I}^{+}}>{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 107) First ionisation potential of \[Be\] and B will be
A)
\[8.8\] and \[8.8\]
done
clear
B)
\[6.6\] and \[6.6\]
done
clear
C)
\[6.6\] and \[8.8\]
done
clear
D)
\[8.8\] and \[6.6\]
done
clear
View Answer play_arrow
question_answer 108) Which of the following electronic configuration will have maximum IP difference between II nd and IIIrd ionisation potential?
A)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}\]
done
clear
C)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}\]
done
clear
D)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{5}}\]
done
clear
View Answer play_arrow
question_answer 109) Which of the following is true for a reaction in which all the products are liquid?
A)
\[\Delta H=\Delta E\]
done
clear
B)
\[\Delta H=\Delta W\]
done
clear
C)
\[\Delta H>\Delta E\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 110) Cell reaction is spontaneous when
A)
\[\Delta {{G}^{o}}\] is negative
done
clear
B)
\[\Delta {{G}^{o}}\] is positive
done
clear
C)
\[\Delta E_{red}^{o}\]is positive
done
clear
D)
\[\Delta E_{red}^{o}\] is negative
done
clear
View Answer play_arrow
question_answer 111) At \[{{27}^{o}}C\]latent heat of fusion of a compound is \[2930\text{ }J/mol,\] then entropy change is
A)
\[9.77J/mol-K\]
done
clear
B)
\[10.77\text{ }J/mol-K\]
done
clear
C)
\[9.07\text{ }J/mol-K\]
done
clear
D)
\[0.977\text{ }J/mol-K\]
done
clear
View Answer play_arrow
question_answer 112) For the reaction,\[{{C}_{2}}{{H}_{5}}OH(l)+3{{O}_{2}}(g)\xrightarrow{{}}2C{{O}_{2}}(g)+3{{H}_{2}}O(l)\] Which one is true?
A)
\[\Delta H=\Delta E-RT\]
done
clear
B)
\[\Delta H=\Delta E+RT\]
done
clear
C)
\[\Delta H=\Delta E+2RT~\]
done
clear
D)
\[\Delta H=\Delta E-2RT~\]
done
clear
View Answer play_arrow
question_answer 113) \[1.1\]moles of A and \[2.2\] moles of B are mixed in a container of one litre volume to obtain the equilibrium \[A+2B\rightleftharpoons 2C+D.\]. At equilibrium 0.2 moles of C are formed. The equilibrium constant for the above reaction is
A)
\[0.001\]
done
clear
B)
\[0.002\]
done
clear
C)
\[0.003\]
done
clear
D)
\[0.004\]
done
clear
View Answer play_arrow
question_answer 114) In which solution/solvent the solubility of \[AgCl\] is minimum?
A)
\[0.01\text{ }M\text{ }NaCl\]
done
clear
B)
\[0.01\text{ }M\,\,CaC{{l}_{2}}\]
done
clear
C)
Pure water
done
clear
D)
\[0.001\text{ }M\,\,AgN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 115) The volume strength of \[1.5\,N\,\,{{H}_{2}}{{O}_{2}}\] solution is
A)
\[8.4\]
done
clear
B)
\[4.8\]
done
clear
C)
\[5.2\]
done
clear
D)
\[8.8\]
done
clear
View Answer play_arrow
question_answer 116) Which one of the following salts gives an acidic solution in water?
A)
\[C{{H}_{3}}COONa\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[C{{H}_{3}}COON{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 117) A cell, with cell constant \[0.4\text{ }c{{m}^{-1}},\] has the resistance of 40 ohm of a \[0.01\text{ }M\]solution of an electrolyte, then the molar conductivity in \[oh{{m}^{-1}}\text{ }c{{m}^{2}}\text{ }mo{{l}^{-1}}\]will be
A)
\[{{10}^{4}}\]
done
clear
B)
\[{{10}^{3}}\]
done
clear
C)
\[{{10}^{2}}\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 118) \[{{H}_{2}}{{O}_{2}}\] on oxidation gives
A)
\[{{O}^{2-}}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[O_{2}^{-}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 119) If the standard electrode potential for the cell \[Zn|Z{{n}^{2+}}(aq)||C{{u}^{2+}}(aq)|Cu\] is \[1.10V\]then the maximum work done by this cell will be
A)
\[-106.15\text{ }kJ\]
done
clear
B)
\[~-212.30\text{ }kJ\]
done
clear
C)
\[-318.45\text{ }kJ\]
done
clear
D)
\[-424.60\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 120) The oxidation states of sulphur in the anions \[SO_{3}^{2-},{{S}_{2}}O_{4}^{2-}\] and \[{{S}_{2}}O_{6}^{2-}\] follow the order
A)
\[{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}<{{S}_{2}}O_{6}^{2-}\]
done
clear
B)
\[SO_{3}^{2-}<{{S}_{2}}O_{4}^{2-}<{{S}_{2}}O_{6}^{2-}\]
done
clear
C)
\[{{S}_{2}}O_{4}^{2-}<{{S}_{2}}O_{6}^{2-}<SO_{3}^{2-}\]
done
clear
D)
\[{{S}_{2}}O_{6}^{2-}<{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 121) \[3A\xrightarrow{{}}2B,\] rate of reaction \[\frac{+d(B)}{dt}\]is equals to
A)
\[-\frac{3}{2}\frac{d(A)}{dt}\]
done
clear
B)
\[-\frac{2}{3}\frac{d(A)}{dt}\]
done
clear
C)
\[-\frac{1}{3}\frac{d(A)}{dt}\]
done
clear
D)
\[+2\frac{d(A)}{dt}\]
done
clear
View Answer play_arrow
question_answer 122) \[2A\xrightarrow{{}}B+C\] It would be a zero order reaction when
A)
the rate of reaction is proportional to square of concentration of A
done
clear
B)
the rate of reaction remains same at any concentration of A
done
clear
C)
the rate remains unchanged at any concentration of B and C
done
clear
D)
the rate of reaction doubles if concentration of B is increased to double
done
clear
View Answer play_arrow
question_answer 123) The activation energy for a simple chemical reaction \[A\xrightarrow{{}}B\]is \[{{E}_{a}}\] in forward direction. The activation energy for reverse reaction
A)
is negative of \[{{E}_{a}}\]
done
clear
B)
is always less than \[{{E}_{a}}\]
done
clear
C)
can be less than or more than \[{{E}_{a}}\]
done
clear
D)
is always double of \[{{E}_{a}}\]
done
clear
View Answer play_arrow
question_answer 124) The reaction \[A\xrightarrow{{}}B\] follows first order kinetics. The time taken for \[0.8\text{ }mole\]of A to produce 0.6 mole of B is\[1\text{ }h\]. What is the time taken for conversion of \[0.9\text{ }mole\]of A to produce \[0.675\text{ }mole\]of B?
A)
\[1h\]
done
clear
B)
\[0.5\text{ }h\]
done
clear
C)
\[0.25\text{ }h\]
done
clear
D)
\[2\text{ }h\]
done
clear
View Answer play_arrow
question_answer 125) Which of the following exhibits highest solubility in water?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[As{{H}_{3}}\]
done
clear
D)
\[Sb{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 126) Chlorine cannot displace
A)
fluorine from \[NaF\]
done
clear
B)
iodine from \[NaI\]
done
clear
C)
bromine from \[NaBr\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 127) Very dilute nitric acid reacts with zinc to form. zinc nitrate and
A)
ammonium nitrate
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[NO\]
done
clear
D)
\[{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 128) The ionic radii of \[{{N}^{3-}},{{O}^{2-}},{{F}^{-}}\] and \[N{{a}^{+}}\] follow the order
A)
\[{{N}^{3-}}>{{O}^{2-}}>{{F}^{-}}>N{{a}^{+}}\]
done
clear
B)
\[{{N}^{3-}}>N{{a}^{+}}>{{O}^{2-}}>{{F}^{-}}\]
done
clear
C)
\[N{{a}^{+}}>{{O}^{2-}}>{{N}^{3-}}>{{F}^{-}}\]
done
clear
D)
\[{{O}^{2-}}>{{F}^{-}}>N{{a}^{+}}>{{N}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 129) The structure of ionic compound with Schottky defects has
A)
same number of cationic and anionic vacancies
done
clear
B)
anionic vacancy and interstitial anion
done
clear
C)
cationic vacancies
done
clear
D)
cationic vacancies and interstitial cation
done
clear
View Answer play_arrow
question_answer 130) If a pentavalent impurity is mixed in the crystal-lattice of germanium then the semiconductor formed will be
A)
p-type semiconductor
done
clear
B)
n-type semiconductor
done
clear
C)
(i) and (ii) both
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 131) A compound formed by elements X and Y crystallises in a cubic structure in which the X atoms are at the corners of a cube and the Y atoms are at the face centres. The formula of the compound is
A)
\[X{{Y}_{3}}\]
done
clear
B)
\[{{X}_{3}}Y\]
done
clear
C)
\[XY\]
done
clear
D)
\[X{{Y}_{2}}\]
done
clear
View Answer play_arrow
question_answer 132) In a face centred cubic lattice, a unit cell a shared equally by how many unit cells?
A)
\[4\]
done
clear
B)
\[2\]
done
clear
C)
\[6\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 133) Acetic acid dissolved in benzene shows molecular weight
A)
\[60\]
done
clear
B)
\[120\]
done
clear
C)
\[180\]
done
clear
D)
\[240\]
done
clear
View Answer play_arrow
question_answer 134) The vapour pressure decreases by \[10\text{ }mm\] of \[Hg\]when mole fraction of solute in a solution is\[0.2\] If the vapour pressure decreases to \[20\text{ }mm\]of \[Hg\]then the mole fraction of solute will be
A)
\[0.2\]
done
clear
B)
\[0.4\]
done
clear
C)
\[0.6\]
done
clear
D)
\[0.8\]
done
clear
View Answer play_arrow
question_answer 135) Volume of \[C{{O}_{2}}\]obtained by the complete decomposition of \[9.85\text{ }g\] \[BaC{{O}_{3}}\] is
A)
\[2.24\text{ }L\]
done
clear
B)
\[1.12L\]
done
clear
C)
\[0.84\text{ }L\]
done
clear
D)
\[0.56\text{ }L\]
done
clear
View Answer play_arrow
question_answer 136) During osmosis, flow of water through a semipermeable membrane is
A)
from both sides of semipermeable membrane with equal flow rate
done
clear
B)
from solution having lower concentration only
done
clear
C)
from solution having higher concentration only
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 137) What is the entropy change (in \[J{{K}^{-1}}\,\,mo{{l}^{-1}}\]) when one mole of ice is converted into water at \[{{0}^{o}}C\]? (The enthalpy change for the conversion of ice to liquid water is \[6.0\text{ }kJ\text{ }mo{{l}^{-1}}\]at\[{{0}^{o}}C\]).
A)
\[20.13\]
done
clear
B)
\[2.013\]
done
clear
C)
\[2.198\]
done
clear
D)
\[21.98\]
done
clear
View Answer play_arrow
question_answer 138) The molar heat capacity of water at constant pressure, is \[75J\,\,{{K}^{-1}}\text{ }mo{{l}^{-1}}\]. When \[1.0\text{ }kJ\]of heat is supplied to \[100\text{ }g\]of water which is free to expand, the increase in temperature of water is
A)
\[1.2K\]
done
clear
B)
\[2.4\text{ }K\]
done
clear
C)
\[4.8\text{ }K\]
done
clear
D)
\[6.6\text{ }K\]
done
clear
View Answer play_arrow
question_answer 139) Which of the following pairs of a chemical reaction is certain to result in a spontaneous reaction?
A)
Endothermic and decreasing disorder
done
clear
B)
Exothermic and increasing disorder
done
clear
C)
Endothermic and increasing disorder
done
clear
D)
Exothermic and decreasing disorder
done
clear
View Answer play_arrow
question_answer 140) Identify the correct statement for change of Gibbs energy for a system \[(\Delta {{G}_{system}})\] at constant temperature and pressure
A)
If \[\Delta {{G}_{system}}=0,\] the system has attained equilibrium
done
clear
B)
If \[\Delta {{G}_{system}}=0,\] the system is still moving in a particular direction
done
clear
C)
If \[\Delta {{G}_{system}}=0,\] the process is not spontaneous
done
clear
D)
If \[\Delta {{G}_{system}}=0,\]the process is spontaneous
done
clear
View Answer play_arrow
question_answer 141) In electrolysis of \[NaCl\]when \[Pt\] electrode is taken then \[{{H}_{2}}\] is liberated at cathode while with \[Hg\]electrode it forms sodium amalgam
A)
\[Hg\] is more inert than \[Pt\]
done
clear
B)
more voltage is required to reduce \[{{H}^{+}}\] at \[Hg\] than at \[Pt\]
done
clear
C)
\[Na\]is dissolved in \[Hg\] while it does not dissolve in \[Pt\]
done
clear
D)
concentration of \[{{H}^{+}}\] ions is larger when P\[Pt\] electrode is taken
done
clear
View Answer play_arrow
question_answer 142) On the basis of the information available from the reaction\[\frac{4}{3}Al+{{O}_{2}}\xrightarrow{{}}\frac{2}{3}A{{l}_{2}}{{O}_{3}},\,\Delta G=-827\,kJ\,mo{{l}^{-1}}\] of \[{{O}_{2}}\], the minimum emf required to carry out an electrolysis of \[A{{l}_{2}}{{O}_{3}}\]is \[(F=96500C\,\,mo{{l}^{-1}})\]
A)
\[2.14V\]
done
clear
B)
\[4.28V\]
done
clear
C)
6.42V
done
clear
D)
\[8.56V\]
done
clear
View Answer play_arrow
question_answer 143) The standard emf of a galvanic cell involving cell reaction with \[n=2\] is found to be \[0.295\text{ }V\]at \[{{25}^{o}}C\]. The equilibrium constant of the reaction would be
A)
\[2.0\times {{10}^{11}}\]
done
clear
B)
\[4.0\times {{10}^{12}}\]
done
clear
C)
\[1.0\times {{10}^{2}}\]
done
clear
D)
\[1.0\times {{10}^{10}}\] (Given,\[F=96500\text{ }C\text{ }mo{{l}^{-1}};\] \[R=8.314\text{ }J{{K}^{-1}}\text{ }mo{{l}^{-1}}\])
done
clear
View Answer play_arrow
question_answer 144) 44. \[4.5g\] of aluminium (at. mass 27 u) is deposited at cathode from \[A{{l}^{3+}}\] solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from \[{{H}^{+}}\]ions in solution by the same quantity of electric charge will be
A)
\[44.8\text{ }L\]
done
clear
B)
\[11.2L\]
done
clear
C)
\[22.4\text{ }L\]
done
clear
D)
\[5.6\text{ }L\]
done
clear
View Answer play_arrow
question_answer 145) The time of completion of \[90%\] of a first order reaction is approximately
A)
1.1 times that of half-life
done
clear
B)
2.2 times that of half-life
done
clear
C)
3.3 times that of half-life
done
clear
D)
4.4 times that of half-life
done
clear
View Answer play_arrow
question_answer 146) When a biochemical reaction is carried out in laboratory from outside of human body in the absence of enzyme than rate of reaction obtained is \[{{10}^{-6}}\] times, then activation energy of reaction in the presence of enzyme is
A)
\[6/RT\]
done
clear
B)
P is required
done
clear
C)
different from, \[{{E}_{a}}\] obtained in laboratory
done
clear
D)
can't say any things
done
clear
View Answer play_arrow
question_answer 147) The rate of reaction between two reactants A and B decreases by a factor of 4 if the concentration of reactant B is doubled. The order of this reaction with respect to reactant B is
A)
\[2\]
done
clear
B)
\[-1\]
done
clear
C)
\[1\]
done
clear
D)
\[-2\]
done
clear
View Answer play_arrow
question_answer 148) In a first-order reaction \[A\xrightarrow{{}}B,\]if fc is rate constant and initial concentration of reactant A is \[0.5\text{ }M\]then the half-life is
A)
\[\frac{In\,\,2}{k}\]
done
clear
B)
\[\frac{0.693}{0.5}\]
done
clear
C)
\[\frac{\log \,2}{2k}\]
done
clear
D)
\[\frac{\log \,2}{k\sqrt{0.5}}\]
done
clear
View Answer play_arrow
question_answer 149) Which ion is colorless?
A)
\[C{{r}^{4+}}\]
done
clear
B)
\[S{{c}^{3+}}\]
done
clear
C)
\[T{{i}^{3+}}\]
done
clear
D)
\[{{V}^{4+}}\]
done
clear
View Answer play_arrow
question_answer 150) Which of the following statement is not correct?
A)
\[La{{(OH)}_{3}}\] is less basic than \[Lu{{(OH)}_{3}}\].
done
clear
B)
In lanthanide series ionic radius of \[L{{u}^{3+}}\] ion decreases.
done
clear
C)
\[La\] is actually an element of transition series rather lanthanide.
done
clear
D)
Atomic radius of \[Zr\] and Hf are same because of lanthanide contraction.
done
clear
View Answer play_arrow
question_answer 151) Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy?
A)
Vanadium \[(Z=23)\]
done
clear
B)
Manganese \[(Z=25)\]
done
clear
C)
Chromium \[(Z=24)\]
done
clear
D)
Iron \[(Z=26)\]
done
clear
View Answer play_arrow
question_answer 152) Which of the following will replace \[B{{r}_{2}}\] from the aqueous solution having bromide ion?
A)
\[C{{l}_{2}}\]
done
clear
B)
\[I_{3}^{\bigcirc -}\]
done
clear
C)
\[{{I}_{2}}\]
done
clear
D)
\[C{{l}^{\bigcirc -}}\]
done
clear
View Answer play_arrow
question_answer 153) If \[2.0\text{ }g\]of a radioactive substance has half-life of 7 days, the half-life of \[1\text{ }g\]sample is
A)
7 days
done
clear
B)
14 days
done
clear
C)
28 days
done
clear
D)
35 days
done
clear
View Answer play_arrow
question_answer 154) \[_{n}{{X}^{m}}\] emit one a-and two P-particles. It is converted into
A)
\[_{n}{{X}^{m-4}}\]
done
clear
B)
\[_{n-1}{{X}^{m-1}}\]
done
clear
C)
\[_{n}{{Z}^{m-4}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 155) \[-{{[NH{{(C{{H}_{2}})}_{6}}NHCO{{(C{{H}_{2}})}_{4}}]}_{n}}-\] is a
A)
addition polymer
done
clear
B)
thermosetting polymer
done
clear
C)
homopolymer
done
clear
D)
copolymer
done
clear
View Answer play_arrow
question_answer 156) Isoprene is a valuable substance for making
A)
propene
done
clear
B)
liquid fuel
done
clear
C)
synthetic rubber
done
clear
D)
petrol
done
clear
View Answer play_arrow
question_answer 157) Enzymes are made up of
A)
edible proteins
done
clear
B)
proteins with specific structure
done
clear
C)
nitrogen containing carbohydrates
done
clear
D)
carbohydrates
done
clear
View Answer play_arrow
question_answer 158) According to the adsorption theory of catalysis. the speed of the reaction increase because
A)
the concentration of reactant molecules at the active centres of the catalyst becomes high due to adsorption
done
clear
B)
in the process of adsorption, the activation energy of the molecules becomes large
done
clear
C)
absorption produces heat which increases the speed of the reaction
done
clear
D)
adsorption lowers the activation energy of the reaction
done
clear
View Answer play_arrow
question_answer 159) In the metallurgy of which of the following cupellation process is used?
A)
Copper
done
clear
B)
Silver
done
clear
C)
Iron
done
clear
D)
Aluminium
done
clear
View Answer play_arrow
question_answer 160) Cryolite is used in the electrolytic extraction of aluminium
A)
to obtain more aluminium
done
clear
B)
to decompose bauxite
done
clear
C)
to protect anodes
done
clear
D)
as a reducing agent
done
clear
View Answer play_arrow
question_answer 161) 18 carat gold contains
A)
\[18%\] gold
done
clear
B)
\[24%\] gold
done
clear
C)
\[75%\] gold
done
clear
D)
\[60%\] gold
done
clear
View Answer play_arrow
question_answer 162) The brass is an alloy of
A)
gold and copper
done
clear
B)
silver and zinc
done
clear
C)
copper and zinc
done
clear
D)
copper and aluminium
done
clear
View Answer play_arrow
question_answer 163) Which of the following metals will not form an amalgam?
A)
Gold
done
clear
B)
Silver
done
clear
C)
Zinc
done
clear
D)
Iron
done
clear
View Answer play_arrow
question_answer 164) Which of the following is pseudo halogen?
A)
\[I{{F}_{7}}\]
done
clear
B)
\[{{(CN)}_{2}}\]
done
clear
C)
\[ICl_{2}^{-}\]
done
clear
D)
\[I_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 165) The element which liberates oxygen gas from water is
A)
\[P\]
done
clear
B)
\[Na\]
done
clear
C)
\[F\]
done
clear
D)
\[I\]
done
clear
View Answer play_arrow
question_answer 166) Which element from group V gives most basic compound with hydrogen?
A)
Nitrogen
done
clear
B)
Bismuth
done
clear
C)
Arsenic
done
clear
D)
Phosphorus
done
clear
View Answer play_arrow
question_answer 167) Which is the weakest acid out of \[HF,\] \[HCl,\] \[HBr\]and\[HI\]?
A)
\[HF\]
done
clear
B)
\[HCl\]
done
clear
C)
\[HBr\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 168) The molecular formula of bleaching powder is
A)
\[CaC{{l}_{2}}\]
done
clear
B)
\[C{{a}_{2}}OC{{l}_{2}}\]
done
clear
C)
\[CaOC{{l}_{2}}\]
done
clear
D)
\[CaC{{l}_{2}}.CaOCl\]
done
clear
View Answer play_arrow
question_answer 169) The strongest oxidising agent amongst \[{{F}_{2}},\] \[C{{l}_{2}};\]\[B{{r}_{2}}\] and \[{{I}_{2}}\] is
A)
\[{{F}_{2}}\]
done
clear
B)
\[{{I}_{2}}\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[B{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 170) Point out in which of the following properties oxygen differs from the rest of the members of its family (Group-VIA)?
A)
High value of ionisation energies
done
clear
B)
Oxidation states (2, 4, 6)
done
clear
C)
Polymorphism
done
clear
D)
Formation of hydrides
done
clear
View Answer play_arrow
question_answer 171) Which of the following properties increases on going down from F to I in group VII-A of the periodic table?
A)
Electronegativity
done
clear
B)
Volatile nature
done
clear
C)
Ionic radius
done
clear
D)
Oxidising power
done
clear
View Answer play_arrow
question_answer 172) The oxidation state of oxygen is zero in
A)
\[CO\]
done
clear
B)
\[{{O}_{3}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 173) A solution of sodium metal in liquid ammonia is a strong reducing agent due to the presence of
A)
sodium atoms
done
clear
B)
sodium hydride
done
clear
C)
sodium amide
done
clear
D)
solvated electron
done
clear
View Answer play_arrow
question_answer 174) A mixture containing \[C{{u}^{2+}}\] and \[N{{i}^{2+}}\] can be separated for identification by
A)
passing \[{{H}_{2}}S\] in acid medium
done
clear
B)
passing \[{{H}_{2}}S\] in alkaline medium
done
clear
C)
passing \[{{H}_{2}}S\] in neutral medium
done
clear
D)
passing \[{{H}_{2}}S\] in dry mixture
done
clear
View Answer play_arrow
question_answer 175) The indicator used in the titration of acetic add with sodium hydroxide for quantitative estimation is
A)
phenolphthalein
done
clear
B)
methyl orange
done
clear
C)
methyl red
done
clear
D)
a mixture of methyl red and methyl orange
done
clear
View Answer play_arrow
question_answer 176) A compound has the empirical formula \[C{{H}_{2}}O\]. Its vapour density is 30. Its molecular formula is
A)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}O\]
done
clear
C)
\[{{C}_{2}}{{H}_{6}}{{O}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}O\]
done
clear
View Answer play_arrow
question_answer 177) The IUPAC name of the compound \[C{{H}_{3}}-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,H-\underset{{{C}_{2}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{3}}\]is
A)
\[2-ethyl-3-methyl\text{ }butane\]
done
clear
B)
\[2,3-dimethyl\text{ }pentane\]
done
clear
C)
\[2-methyl-3-ethyl\text{ }butane\]
done
clear
D)
\[3,4-dimethyl\text{ }pentane\]
done
clear
View Answer play_arrow
question_answer 178) Out of the following fractions of petroleum the one having the lowest boiling point is
A)
kerosene oil
done
clear
B)
diesel oil
done
clear
C)
gasoline
done
clear
D)
heavy oil
done
clear
View Answer play_arrow
question_answer 179) Coordination number of Fe in the complexes \[{{[Fe{{(CN)}_{6}}]}^{4-}},{{[Fe{{(CN)}_{6}}]}^{3-}}\]and \[{{[FeC{{l}_{4}}]}^{-}}\] would be respectively
A)
\[6,3,4\]
done
clear
B)
\[6,6,4\]
done
clear
C)
\[2,3,3\]
done
clear
D)
\[6,4,6\]
done
clear
View Answer play_arrow
question_answer 180) Methoxy methane and ethanol are
A)
functional isomers
done
clear
B)
chain isomers
done
clear
C)
optical isomers
done
clear
D)
geometrical isomers
done
clear
View Answer play_arrow
question_answer 181) Most stable ion is
A)
\[{{C}_{6}}{{H}_{5}}CH_{2}^{+}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}-{{C}^{+}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}C{{H}^{+}}\]
done
clear
D)
\[C{{H}_{3}}CH_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 182) Formation of 2-butene as major product by dehydration of 2-butanol is according to
A)
Markownikoff?s rule
done
clear
B)
Saytzeffrule
done
clear
C)
Peroxide effect
done
clear
D)
Anti-Markownikoff?s rule
done
clear
View Answer play_arrow
question_answer 183) \[64\text{ }g\]of an organic compound contains \[24\text{ }g\]of carbon, \[\text{8 }g\] of hydrogen and the rest oxygen. The empirical formula of the compound is
A)
\[C{{H}_{2}}O\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}O\]
done
clear
C)
\[C{{H}_{4}}O\]
done
clear
D)
\[{{C}_{2}}{{H}_{8}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 184) \[[Pt{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]B{{r}_{2}}\] and \[[Pt{{(N{{H}_{3}})}_{4}}B{{r}_{2}}]C{{l}_{2}}\] are related to each other as
A)
optical isomers
done
clear
B)
coordinate isomers
done
clear
C)
ionisation isomers
done
clear
D)
linkage isomers
done
clear
View Answer play_arrow
question_answer 185) Which one of the following substances is used as an anti-knock compound?
A)
Tetra ethyl lead
done
clear
B)
Lead tetrachloride
done
clear
C)
Lead acetate
done
clear
D)
Ethyl acetate
done
clear
View Answer play_arrow
question_answer 186) How many isomeric butanes are there?
A)
\[2\]
done
clear
B)
\[3\]
done
clear
C)
\[4\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 187) Ethene gives with acidic \[KMn{{O}_{4}}\] solution
A)
ethylene glycol
done
clear
B)
ethylene oxide
done
clear
C)
formaldehyde
done
clear
D)
acetaldehyde
done
clear
View Answer play_arrow
question_answer 188) Which of the following is an organo-metallic compound?
A)
Lithium ethoxide
done
clear
B)
Ethyl lithium
done
clear
C)
Lithium acetate
done
clear
D)
Lithium carbide
done
clear
View Answer play_arrow
question_answer 189) When phenol is distilled with \[Zn\] dust it gives
A)
benzaldehyde
done
clear
B)
benzoic acid
done
clear
C)
toluene
done
clear
D)
benzene
done
clear
View Answer play_arrow
question_answer 190) Ethane is formed by the reaction of methyl iodide and sodium metal in dry ether solution. This reaction is known as
A)
Clemmensen's reduction
done
clear
B)
Kolbe's reaction
done
clear
C)
Wurtz reaction
done
clear
D)
Cannizaro's reaction
done
clear
View Answer play_arrow
question_answer 191) At normal room temperature iodoform is
A)
volatile liquid
done
clear
B)
thick viscous liquid
done
clear
C)
gas
done
clear
D)
solid
done
clear
View Answer play_arrow
question_answer 192) The compound added to prevent chloroform to form phosgene gas (poisonous gas) is
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 193) The formula of freon-12 is
A)
\[CCl{{F}_{3}}\]
done
clear
B)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
C)
\[CC{{l}_{2}}{{F}_{2}}\]
done
clear
D)
\[C{{H}_{2}}{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 194) The reaction of benzaldehyde with alkali gives
A)
phenol + sodium benzoate
done
clear
B)
benzene + benzyl alcohol
done
clear
C)
benzyl alcohol + sodium benzoate
done
clear
D)
phenol + benzene
done
clear
View Answer play_arrow
question_answer 195) The reaction \[{{C}_{2}}{{H}_{5}}ONa+Br{{C}_{2}}{{H}_{5}}\xrightarrow{{}}\]\[{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}+NaBr\]is called
A)
Frank land reaction
done
clear
B)
Wurtz reaction
done
clear
C)
Williamson's synthesis
done
clear
D)
Cannizaro reaction
done
clear
View Answer play_arrow
question_answer 196) Diethyl ether on heating with cone. HI gives two moles of
A)
ethyl iodide
done
clear
B)
ethanol
done
clear
C)
iodoform
done
clear
D)
methyl iodide
done
clear
View Answer play_arrow
question_answer 197) Positive Fehling test is shown by
A)
acetaldehyde
done
clear
B)
acetone
done
clear
C)
benzaldehyde
done
clear
D)
acetophenone
done
clear
View Answer play_arrow
question_answer 198) \[RCON{{H}_{2}}+B{{r}_{2}}+KOH\xrightarrow{{}}\text{Amine}\]This reaction is
A)
Carbyl amine reaction
done
clear
B)
Mustard oil reaction
done
clear
C)
Hofmann bromide reaction
done
clear
D)
Cannizzaro reaction
done
clear
View Answer play_arrow
question_answer 199) Which of the following is reducing agent?
A)
\[LiAl{{H}_{4}}\]
done
clear
B)
\[Zn+HCl\]
done
clear
C)
\[Sn+HCl\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 200) Reduction of an aldehyde gives
A)
primary alcohol
done
clear
B)
monocarboxylic acid
done
clear
C)
secondary alcohol
done
clear
D)
tertiary alcohol
done
clear
View Answer play_arrow
question_answer 201) Cells of the epithelial tissue rest on a basement membrane, which is made up of
A)
mono saccharides
done
clear
B)
muco polysaccharides
done
clear
C)
disaccharides
done
clear
D)
lipids
done
clear
View Answer play_arrow
question_answer 202) The cells of a tissue are similar in
A)
structure
done
clear
B)
function
done
clear
C)
origin
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 203) The intercellular matrix is negligible or absent in which of the following tissues?
A)
Connective tissue
done
clear
B)
Epithelial tissue
done
clear
C)
Muscular tissue
done
clear
D)
Cardiac tissue
done
clear
View Answer play_arrow
question_answer 204) The basement membrane acts as
A)
plasma membrane
done
clear
B)
plasmalemma
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 205) The filaments arising from desmosomes are called
A)
tonofibril
done
clear
B)
tonofilament
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 206) Pseudo stratified epithelium is always
A)
single layered
done
clear
B)
double layered
done
clear
C)
multilayered
done
clear
D)
uncertain
done
clear
View Answer play_arrow
question_answer 207) Most of the glands of the body are of
A)
holocrine type
done
clear
B)
merocrine type
done
clear
C)
apocrine type
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 208) Pyruvic acid before combining with oxaloaceric acid of Krebs' cycle becomes
A)
lactic acid
done
clear
B)
acetoacetic acid
done
clear
C)
cis-aconitic acid
done
clear
D)
acetyl Co-A
done
clear
View Answer play_arrow
question_answer 209) Dissociation curve shifts to the right when
A)
\[C{{O}_{2}}\] concentration decreases
done
clear
B)
\[C{{O}_{2}}\]concentration increases
done
clear
C)
\[{{O}_{2}}\]concentration decreases
done
clear
D)
\[\text{C}{{\text{l}}^{-}}\] concentration increases
done
clear
View Answer play_arrow
question_answer 210) Which of the following glands is present in frog's skin and not in rabbit's skin?
A)
Sebaceous gland
done
clear
B)
Sweat gland
done
clear
C)
Mucous gland
done
clear
D)
Moll's gland
done
clear
View Answer play_arrow
question_answer 211) Cutaneous glands are
A)
gastric glands
done
clear
B)
sebaceous glands
done
clear
C)
thyroid gluids
done
clear
D)
thymus glands
done
clear
View Answer play_arrow
question_answer 212) Teeth are absent in
A)
fishes
done
clear
B)
reptiles
done
clear
C)
frogs
done
clear
D)
turtle
done
clear
View Answer play_arrow
question_answer 213) Ivory of teeth is
A)
enamel
done
clear
B)
dentine
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 214) In the gut of a mammal, haustra and taeniae are the parts of
A)
rectum
done
clear
B)
colon
done
clear
C)
caecum
done
clear
D)
ileum
done
clear
View Answer play_arrow
question_answer 215) Space between incisor and cheek teeth in rabbit is
A)
diapause
done
clear
B)
diastema
done
clear
C)
dialysis
done
clear
D)
diapedesis
done
clear
View Answer play_arrow
question_answer 216) Taste buds are absent in
A)
foliate papillae
done
clear
B)
circumvallate papillae
done
clear
C)
fungiform papillae
done
clear
D)
fili form papillae
done
clear
View Answer play_arrow
question_answer 217) Which one respires through gills ?
A)
Crocodile
done
clear
B)
Whale
done
clear
C)
Frog
done
clear
D)
Prawn
done
clear
View Answer play_arrow
question_answer 218) Fish brought out of water dies because of
A)
absence of pressure
done
clear
B)
inability to respire
done
clear
C)
inability to feed
done
clear
D)
rise in temperature
done
clear
View Answer play_arrow
question_answer 219) Skin is an accessory organ of respiration in
A)
human
done
clear
B)
frog
done
clear
C)
rabbit
done
clear
D)
lizard
done
clear
View Answer play_arrow
question_answer 220) In Pheretima, lymph glands produce
A)
phagocytic cells
done
clear
B)
lymphocytic cells
done
clear
C)
amoebocytic cells
done
clear
D)
oxyntic cells
done
clear
View Answer play_arrow
question_answer 221) How many lateral hearts are in Pheretima
A)
4
done
clear
B)
8
done
clear
C)
16
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 222) Four pairs of pulsatile heart in Pheretima are located in segments
A)
7, 9, 12 and 13
done
clear
B)
11,14, 17 and 18
done
clear
C)
10,13,16 and 17
done
clear
D)
4, 5,10 and 13
done
clear
View Answer play_arrow
question_answer 223) Open circulatory system is observed in
A)
cockroach
done
clear
B)
frog
done
clear
C)
fish
done
clear
D)
reptiles
done
clear
View Answer play_arrow
question_answer 224) Single circuit circulatory system is characteristic of
A)
fishes
done
clear
B)
amphibians
done
clear
C)
aves
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 225) Which of the following are uricotelic animals ?
A)
Rohu and frog
done
clear
B)
Lizard and crow
done
clear
C)
Camel and frog
done
clear
D)
Earthworm and eagle
done
clear
View Answer play_arrow
question_answer 226) In gout patients, high level of which of the following is found in blood?
A)
Urea
done
clear
B)
Uric add
done
clear
C)
Cholesterol
done
clear
D)
Amino acid
done
clear
View Answer play_arrow
question_answer 227) Excretion of nitrogenous waste products in semisolid form occurs in
A)
ureotelic animals
done
clear
B)
ammonotelic animals
done
clear
C)
uricotelic animals
done
clear
D)
ammonites
done
clear
View Answer play_arrow
question_answer 228) The phenomenon, which represents terrestrial mode (dry habitat) of life, is
A)
ammonotelism
done
clear
B)
ureotelism
done
clear
C)
uricotelism
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 229) Aquatic reptiles are
A)
ammonotelic
done
clear
B)
ureotelic over land
done
clear
C)
ureotelic
done
clear
D)
ureotelic in water
done
clear
View Answer play_arrow
question_answer 230) Ammonia is the chief excretory substance in
A)
camel and whale
done
clear
B)
cartilaginous fishes
done
clear
C)
whale and tortoise
done
clear
D)
fresh water fishes
done
clear
View Answer play_arrow
question_answer 231) The chief nitrogenous waste in urine of rabbit or terrestrial mammals is
A)
urea
done
clear
B)
uric acid
done
clear
C)
ammonia
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 232) The correct sequence of meninges from outer to the inner side is
A)
Arachnoid-piamater-duramater
done
clear
B)
Arachnoid-duramater-piamater
done
clear
C)
Piamater-arachnoid-duramater
done
clear
D)
Duramater-arachnoid-piamater
done
clear
View Answer play_arrow
question_answer 233) On the basis of body organization, animals are grouped as
A)
Metazoa and Eumetazoa
done
clear
B)
Protozoa and Parazoa
done
clear
C)
Parazoa and Metazoa
done
clear
D)
Protozoa and Metazoa
done
clear
View Answer play_arrow
question_answer 234) Which one of the following cells form the lining of brain ?
A)
Ependymal cells
done
clear
B)
Neurons
done
clear
C)
Schwann cells
done
clear
D)
Neurilemma
done
clear
View Answer play_arrow
question_answer 235) Cerebrospinal fluid is produced by
A)
ependymal cells
done
clear
B)
choroid plexuses
done
clear
C)
neuroglial cells
done
clear
D)
neurons
done
clear
View Answer play_arrow
question_answer 236) The ventricles of the brain are filled with
A)
cerebrospinal fluid
done
clear
B)
lymph
done
clear
C)
blood
done
clear
D)
amniotic fluid
done
clear
View Answer play_arrow
question_answer 237) Which one of the following phenomenon is not common to both oestrous and menstrual cycles?
A)
Growth of ovarian follicles under the influence of FSH and LH
done
clear
B)
Formation of corpus luteum in the ovarian follicles.
done
clear
C)
Sexual receptivity limited to a definite period in the cycle
done
clear
D)
Regulation of cycles by estrogen and progesterone
done
clear
View Answer play_arrow
question_answer 238) For ovulation in reflex ovulators
A)
coitus is not necessary
done
clear
B)
coitus is necessary
done
clear
C)
plenty of food is necessary
done
clear
D)
plenty of food is not necessary
done
clear
View Answer play_arrow
question_answer 239) Secondary sexual characters in females develop in response to hormone
A)
relaxin
done
clear
B)
progesterone
done
clear
C)
estrogen
done
clear
D)
gonadotropin
done
clear
View Answer play_arrow
question_answer 240) The period of restoration of uterine epithelium is called
A)
menstrual phase
done
clear
B)
proliferative phase
done
clear
C)
secretory or luteal phase
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 241) Which one of the following exhibits menstruation ?
A)
Tiger
done
clear
B)
Chimpanzee
done
clear
C)
Civet cat
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 242) Glenoid cavity is found in
A)
pelvic girdle
done
clear
B)
pectoral girdle
done
clear
C)
skull
done
clear
D)
humerus
done
clear
View Answer play_arrow
question_answer 243) Axis vertebra is identified by
A)
olecranon process
done
clear
B)
odontoid process
done
clear
C)
sigmoid notch
done
clear
D)
odontoblast
done
clear
View Answer play_arrow
question_answer 244) When the intensity of light is low during night the light is detected by
A)
rods
done
clear
B)
cones
done
clear
C)
lens
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 245) Colourblindness results from
A)
absence of rods
done
clear
B)
absence of cones
done
clear
C)
absence of eyelids
done
clear
D)
inverted retina
done
clear
View Answer play_arrow
question_answer 246) Who is the "Father of Endocrinology" ?
A)
Whittaker
done
clear
B)
Einthoven
done
clear
C)
Pasteur
done
clear
D)
T Addison
done
clear
View Answer play_arrow
question_answer 247) Steroid is a
A)
thyroid acid
done
clear
B)
vitamin-A
done
clear
C)
cholesterol
done
clear
D)
easter and fatty acid
done
clear
View Answer play_arrow
question_answer 248) The feedback control mechanism is related with
A)
bile secretion
done
clear
B)
\[\text{HCl}\]secretion
done
clear
C)
hormonal secretion
done
clear
D)
hering breuer reflex
done
clear
View Answer play_arrow
question_answer 249) All functions of the body are regulated and integrated by
A)
respiratory system
done
clear
B)
digestive system
done
clear
C)
neuroendocrine system
done
clear
D)
excretory system
done
clear
View Answer play_arrow
question_answer 250) Which of the following does not secrete any hormone ?
A)
Ovary
done
clear
B)
Testis
done
clear
C)
Spleen/liver
done
clear
D)
Pancreas
done
clear
View Answer play_arrow
question_answer 251) The name second messenger is given to
A)
ATP
done
clear
B)
Cyclic-AMP
done
clear
C)
GTP
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 252) Endocrine glands produce or action of endocrine gland is mediated through
A)
hormones
done
clear
B)
enzymes
done
clear
C)
minerals
done
clear
D)
vitamins
done
clear
View Answer play_arrow
question_answer 253) The word 'hormone' means
A)
to move
done
clear
B)
to exdte
done
clear
C)
to initiate
done
clear
D)
to increase
done
clear
View Answer play_arrow
question_answer 254) In which of the following organisms hormones are normally absent?
A)
Monkey
done
clear
B)
Cat
done
clear
C)
Cockroach
done
clear
D)
Bacteria
done
clear
View Answer play_arrow
question_answer 255) Which of the following vitamins is Necessary for blood clotting ?
A)
Vitamin-A
done
clear
B)
Vitamin-E
done
clear
C)
Vitamin-C
done
clear
D)
Vitamin-K
done
clear
View Answer play_arrow
question_answer 256) Deficiency of vitamin-'C' causes
A)
beri beri
done
clear
B)
rickets
done
clear
C)
scurvy
done
clear
D)
night blindness
done
clear
View Answer play_arrow
question_answer 257) The hardest substance of vertebrate body is
A)
keratin
done
clear
B)
enamel
done
clear
C)
dentine
done
clear
D)
chondrin
done
clear
View Answer play_arrow
question_answer 258) The term blastocyst is applied to the blastula of which one of the following ?
A)
Kangaroo
done
clear
B)
Platypus
done
clear
C)
Monkey
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 259) Substances synthesized during growth are
A)
protoplasmic
done
clear
B)
apoplasmic
done
clear
C)
Both [a] and [b]
done
clear
D)
nucleic acid
done
clear
View Answer play_arrow
question_answer 260) Growth in the first 10-13 years of age is controlled by
A)
somatotrophic hormone
done
clear
B)
thyroxin
done
clear
C)
thymosin
done
clear
D)
gonadotropic hormone
done
clear
View Answer play_arrow
question_answer 261) In old age, there is decrease in amount of urine output and difficulty in micturition it is due to
A)
increase in number of nephrons
done
clear
B)
increase in glomerular filtration
done
clear
C)
decrease in number of nephrons and glomerular filtration
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 262) Growth is an irreversible process seen at all organizational levels. It consists of
A)
organ growth
done
clear
B)
subcellular growth
done
clear
C)
cell growth
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 263) When growth takes place only by increase in volume of existing cells it is called as
A)
auxetic growth
done
clear
B)
multiplicative growth
done
clear
C)
accretionary growth
done
clear
D)
differential growth
done
clear
View Answer play_arrow
question_answer 264) The following cells cannot be grown under tissue culture conditions
A)
hela cells
done
clear
B)
leucocytes
done
clear
C)
kidney cells
done
clear
D)
nerve cells
done
clear
View Answer play_arrow
question_answer 265) Growth in most warm blooded vertebrates is
A)
determinate
done
clear
B)
indeterminate
done
clear
C)
uncontrolled
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 266) Wilkins X-ray diffraction showed the diameter of the DNA helix as
A)
\[10\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[20\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[30\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[40\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 267) The number of autosomes in man is
A)
22 pairs
done
clear
B)
11 pairs
done
clear
C)
43 pairs
done
clear
D)
23 pairs
done
clear
View Answer play_arrow
question_answer 268) A normal metaphase chromosome with a middle centromere is
A)
metacentric
done
clear
B)
sub-metacentric
done
clear
C)
acrocentric
done
clear
D)
telocentric
done
clear
View Answer play_arrow
question_answer 269) Lampbrush chromosomes are found inside
A)
salivary glands of Drosophila
done
clear
B)
salivary glands of silk moth
done
clear
C)
oocytes of frog
done
clear
D)
nucleus of man
done
clear
View Answer play_arrow
question_answer 270) Depending upon size and centromere position the 46 chromosomes have been divided into a number of groups
A)
6
done
clear
B)
5
done
clear
C)
7
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 271) Chromosome number is
A)
fixed for a species
done
clear
B)
fixed for an ecosystem
done
clear
C)
fixed for a community
done
clear
D)
fixed for a biosphere
done
clear
View Answer play_arrow
question_answer 272) The twenty third pair of chromosomes in man is known as
A)
chromatid
done
clear
B)
heterosome
done
clear
C)
autosome
done
clear
D)
gene
done
clear
View Answer play_arrow
question_answer 273) The careers of hereditary material are
A)
chromosomes
done
clear
B)
gene
done
clear
C)
gametes
done
clear
D)
gametocytes
done
clear
View Answer play_arrow
question_answer 274) In man, the normal number of chromosomes are
A)
42
done
clear
B)
44
done
clear
C)
46
done
clear
D)
48
done
clear
View Answer play_arrow
question_answer 275) The genetic material of virus is
A)
DNA
done
clear
B)
RNA
done
clear
C)
Both [a] and [b]
done
clear
D)
DNA or RNA
done
clear
View Answer play_arrow
question_answer 276) The chromosomes as thread like structures in nucleus was first described by
A)
Mendel
done
clear
B)
Strasburger
done
clear
C)
Darwin
done
clear
D)
Levitzky
done
clear
View Answer play_arrow
question_answer 277) Genes are made up of
A)
histones
done
clear
B)
lipoproteins
done
clear
C)
polynucleotides
done
clear
D)
hydrocarbons
done
clear
View Answer play_arrow
question_answer 278) The function of chromosomes of carrying the genetic information from one cell generation to another is performed by
A)
RNA
done
clear
B)
DNA
done
clear
C)
histones
done
clear
D)
calcium
done
clear
View Answer play_arrow
question_answer 279) In chromosomes, the material controlling heredity is
A)
DNA
done
clear
B)
histones
done
clear
C)
chromocentres
done
clear
D)
RNA
done
clear
View Answer play_arrow
question_answer 280) Sex ratio in a species indicates the numerical relation between the number of
A)
male and female producing gametes
done
clear
B)
black and white
done
clear
C)
unisexual and bisexual
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 281) The aggregate of processes that determine the size and composition of any population is called
A)
population dispersal
done
clear
B)
population dynamics
done
clear
C)
population explosion
done
clear
D)
population density
done
clear
View Answer play_arrow
question_answer 282) Intracellular digestion is found in the
A)
protozoans only
done
clear
B)
porifers only
done
clear
C)
in some coelenterates only
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 283) Radial symmetry is usually exhibited in an which
A)
have one opening of alimentary canal
done
clear
B)
have colliery mode of feeding
done
clear
C)
are attached to the substratum
done
clear
D)
live in water
done
clear
View Answer play_arrow
question_answer 284) Absence of excretory organs, great power regeneration and exclusively marine animals belong to the phylum
A)
Mollusca
done
clear
B)
Echinodermata
done
clear
C)
Fishes
done
clear
D)
Arthropoda
done
clear
View Answer play_arrow
question_answer 285) Select the correct statement
A)
birds are poikilothermic
done
clear
B)
flatworms are coelomic animal
done
clear
C)
earthworm is metameric ally segmented
done
clear
D)
fishes are radially symmetrical
done
clear
View Answer play_arrow
question_answer 286) Characteristic features such as four-chambered heart, feather and pneumatic bone is applicable to the class of vertebrate
A)
Cyclostomata
done
clear
B)
Aves
done
clear
C)
Reptilia
done
clear
D)
Mammalia
done
clear
View Answer play_arrow
question_answer 287) Which of the following animals is predaceous
A)
Pheretima
done
clear
B)
Nereis
done
clear
C)
Hirudinaria
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 288) Taxon is the
A)
name of a taxonomy journal
done
clear
B)
name of a branch of taxonomy
done
clear
C)
a rank of classification as phylum, class, order, species
done
clear
D)
group of similarly constituted organisms
done
clear
View Answer play_arrow
question_answer 289) Which of the following is the oldest living fossil?
A)
Architeuthis
done
clear
B)
Neopilina
done
clear
C)
Nautilus
done
clear
D)
Limulus
done
clear
View Answer play_arrow
question_answer 290) The non-nucleated, unicellular organisms of Whittaker's (1969) classification are included in the kingdom
A)
Protista
done
clear
B)
Monera
done
clear
C)
Animalia
done
clear
D)
Plantae
done
clear
View Answer play_arrow
question_answer 291) In human evolution, which is most recent?
A)
Middle paleolithic
done
clear
B)
Upper Paleolithic
done
clear
C)
Neolithic
done
clear
D)
Mesolithic
done
clear
View Answer play_arrow
question_answer 292) The concept of sudden genetic change, which breeds true in a species is represented as
A)
inheritance of acquired characters
done
clear
B)
natural selection
done
clear
C)
law of inheritance
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 293) Silkworm is a
A)
fly
done
clear
B)
worm
done
clear
C)
moth
done
clear
D)
beetle
done
clear
View Answer play_arrow
question_answer 294) Who is referred to as father of pearl industry ?
A)
Iwanovsky
done
clear
B)
Louis Pasteur
done
clear
C)
Kokichi Mikimoto
done
clear
D)
Harvey
done
clear
View Answer play_arrow
question_answer 295) The biological name of the Java man is
A)
Homo erectus
done
clear
B)
Homo sapiens
done
clear
C)
Homo habilis
done
clear
D)
Pithecanthropus erectus
done
clear
View Answer play_arrow
question_answer 296) plasmodium in man is in no cleated by
A)
Anopheles male
done
clear
B)
Anopheles female
done
clear
C)
Both [a] and [b]
done
clear
D)
Culex female
done
clear
View Answer play_arrow
question_answer 297) Smoking addiction is harmful because it produces polycyclic aromatic hydrocarbons which cause
A)
reduction in oxygen transport
done
clear
B)
increase in blood pressure
done
clear
C)
cancer
done
clear
D)
retardation of growth of foetus
done
clear
View Answer play_arrow
question_answer 298) Alcohol addiction is harmful because it causes
A)
protein depositions in liver
done
clear
B)
deposition of extra fat in liver
done
clear
C)
rise in blood sugar level
done
clear
D)
cancer
done
clear
View Answer play_arrow
question_answer 299) In which state is the Kanha National Park?
A)
Rajasthan
done
clear
B)
Uttar Pradesh
done
clear
C)
Madhya Pradesh
done
clear
D)
Assam
done
clear
View Answer play_arrow
question_answer 300) The lion tailed monkeys Malaca silenus are found only in these regions
A)
Kaziranga and other pans of Assam
done
clear
B)
Eastern ghats and Chennai
done
clear
C)
Western ghats including Travancore Mysore
done
clear
D)
Himalayan mountains
done
clear
View Answer play_arrow
question_answer 301) The resolution of light microscope could be improved to 1.5 times by using.
A)
Red light and immersion oil between the specimen and objective
done
clear
B)
Blue light and glycerine between the specimen and objective
done
clear
C)
Blue light and immersion oil between specimen and objective
done
clear
D)
Day light and air between specimen and objective
done
clear
View Answer play_arrow
question_answer 302) Separation of proteins on the basis of their being polyelectrolytes can be brought about by
A)
centrifugation
done
clear
B)
electrophoresis
done
clear
C)
chromatography
done
clear
D)
crystallography
done
clear
View Answer play_arrow
question_answer 303) Taylor (1958) studied chromosome replication by using radioactive
A)
uridine
done
clear
B)
thymidine
done
clear
C)
histones
done
clear
D)
phosphate
done
clear
View Answer play_arrow
question_answer 304) The amount of which of the following elements greatest in protoplasm
A)
hydrogen
done
clear
B)
oxygen
done
clear
C)
nitrogen
done
clear
D)
carbon
done
clear
View Answer play_arrow
question_answer 305) Normal pH of protoplasm is
A)
7.8
done
clear
B)
6.8
done
clear
C)
5
done
clear
D)
6.5
done
clear
View Answer play_arrow
question_answer 306) The theory strongly advocating the morphological nature of protoplasm is
A)
granular theory of Altmann
done
clear
B)
fibrical theory of Flemming
done
clear
C)
nuclear theory of Altmann
done
clear
D)
colloidal theory of Wilson Fischer and Hardy
done
clear
View Answer play_arrow
question_answer 307) In rotation type of cyclosis, the cytoplasmic matrix flows in
A)
one direction
done
clear
B)
two opposite directions
done
clear
C)
different directions
done
clear
D)
side wise
done
clear
View Answer play_arrow
question_answer 308) The function of centrosome is
A)
to inhibit cell division
done
clear
B)
to initiate cell division
done
clear
C)
to increase protein synthesis
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 309) At which stage of mitosis, chromatids separate and start moving towards poles?
A)
Prophase
done
clear
B)
Metaphase
done
clear
C)
Anaphase
done
clear
D)
Telophase
done
clear
View Answer play_arrow
question_answer 310) Micro bodies differ from lysosomes in that
A)
micro bodies are surrounded by a single unit membrane, while lysosome membrane is double
done
clear
B)
micro bodies are surrounded by double membrane, while lysosome membrane is single unit
done
clear
C)
micro bodies contain lytic enzymes, while lysosomes do not
done
clear
D)
lysosomes contain lytic enzymes, while micro bodies do not
done
clear
View Answer play_arrow
question_answer 311) The haploid complement of chromosomes of an organism constitutes
A)
genome
done
clear
B)
genotype
done
clear
C)
phenotype
done
clear
D)
genetic system
done
clear
View Answer play_arrow
question_answer 312) The cross used to ascertain whether the plant is homozygous or heterozygous is
A)
linkage cross
done
clear
B)
reciprocal cross
done
clear
C)
test cross
done
clear
D)
monohybrid cross
done
clear
View Answer play_arrow
question_answer 313) The dihybrid ratio in \[{{F}_{2}}\] generation is
A)
1: 1: 1 : 1
done
clear
B)
2 : 1: 2
done
clear
C)
3 :1
done
clear
D)
9 : 3 : 3 :1
done
clear
View Answer play_arrow
question_answer 314) Which of the following trait of garden pea studied by Mendel was recessive feature?
A)
Round seed shape
done
clear
B)
Axial flower position
done
clear
C)
Green seed colour
done
clear
D)
Green pod colour
done
clear
View Answer play_arrow
question_answer 315) Which of the following scientist rediscovered Mendel's theory ?
A)
T H Morgan
done
clear
B)
W Bateson
done
clear
C)
E Strasburger
done
clear
D)
E Von Tschermak
done
clear
View Answer play_arrow
question_answer 316) Which of the following is an example of pleiotropic gene ?
A)
Thalassemia
done
clear
B)
Haemophilia
done
clear
C)
Colour blindness
done
clear
D)
Sickle cell anaemia
done
clear
View Answer play_arrow
question_answer 317) By double fertilization, is formed
A)
endosperm
done
clear
B)
megaspore
done
clear
C)
seed
done
clear
D)
fruit
done
clear
View Answer play_arrow
question_answer 318) In split genes, the coding sequences are called
A)
cistrons
done
clear
B)
operons
done
clear
C)
exons
done
clear
D)
introns
done
clear
View Answer play_arrow
question_answer 319) Extra nuclear genes occur in
A)
plastids and inherited
done
clear
B)
plasmid and not inherited
done
clear
C)
mitochondria and inherited by male
done
clear
D)
mitochondria and inherited by female
done
clear
View Answer play_arrow
question_answer 320) How many different types of genetically different gametes will be produced by a heterozygous plant having the genotype AA Bb Cc ?
A)
Two
done
clear
B)
Four
done
clear
C)
Six
done
clear
D)
Nine
done
clear
View Answer play_arrow
question_answer 321) Drosophila melanogaster has
A)
2 pairs of autosomes and 1 pair of as chromosomes
done
clear
B)
3 pairs of autosomes and 1 pair of as chromosomes
done
clear
C)
1 pair of autosome and 3 pairs of sea chromosomes
done
clear
D)
2 pairs of autosomes and 2 pairs of sea chromosomes
done
clear
View Answer play_arrow
question_answer 322) The four daughter cells derived from a single meiosis differ from each other due to
A)
difference in chromosome number
done
clear
B)
crossing over only
done
clear
C)
independent assortment of chromosomes
done
clear
D)
crossing over as well as independent assortment of chromosomes
done
clear
View Answer play_arrow
question_answer 323) In which kingdom, would you classify the archaea and nitrogen fixing organisms, if the five kingdom system of classification is used ?
A)
Plantae
done
clear
B)
Fungi
done
clear
C)
Protista
done
clear
D)
Monera
done
clear
View Answer play_arrow
question_answer 324) Which one of the following is correct for structure of cell wall of fungi and bacteria?
A)
Both have glycopeptide
done
clear
B)
Both are made up of N-acetyl glycosamine
done
clear
C)
Both are made up of N-acetyl muramic acid
done
clear
D)
Both are made up of chitin
done
clear
View Answer play_arrow
question_answer 325) Muramic acid is present in the cell wall of
A)
bacteria
done
clear
B)
green algae
done
clear
C)
red algae
done
clear
D)
Rhizopus
done
clear
View Answer play_arrow
question_answer 326) Most of the bacteria can tolerate high temperature due to
A)
type of cell wall
done
clear
B)
cell organization
done
clear
C)
homopolar bonds in their proteins
done
clear
D)
absence of phospholipids in their walls
done
clear
View Answer play_arrow
question_answer 327) For reproduction, endospores are formed in the following genera
A)
Bacillus and Clostridium
done
clear
B)
Mucor and Bacillus
done
clear
C)
Mono coccus and Clostridium
done
clear
D)
Saccharomyces and Clostridium
done
clear
View Answer play_arrow
question_answer 328) Plasmids are ideal vectors for gene cloning as they
A)
can be multiplied in a laboratory using enzymes
done
clear
B)
can be multiplied by culturing
done
clear
C)
are self-replicating
done
clear
D)
replicate freely outside the bacterial cells
done
clear
View Answer play_arrow
question_answer 329) Root nodules for nitrogen fixation in non leguminous trees are produced by species of the genus
A)
Rhizobium
done
clear
B)
Azotobacter
done
clear
C)
Frankia
done
clear
D)
Thiobacillus
done
clear
View Answer play_arrow
question_answer 330) Activity of one of the following bacteria is very helpful in the preparation and flavouring of tea leaves
A)
Bacillus subtilis
done
clear
B)
Bacillus megatherium
done
clear
C)
Bacillus aceti
done
clear
D)
Bacillus radicicola
done
clear
View Answer play_arrow
question_answer 331) Why surgical instruments are boiled in water before use?
A)
For killing the pathogens present on them
done
clear
B)
So that doctors can use them easily
done
clear
C)
Provides pleasure to the patient
done
clear
D)
All the saprophytes die on the operative surface
done
clear
View Answer play_arrow
question_answer 332) Pasteurization of milk involves heating for
A)
60 minute at about
done
clear
B)
60 minute at about
done
clear
C)
30 minute at about
done
clear
D)
30 minute at about
done
clear
View Answer play_arrow
question_answer 333) Which kingdom incorporates photoautotrophs, chemoautotrophs and heterotrophs?
A)
Monera
done
clear
B)
Protista
done
clear
C)
Plantae
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 334) Blue-green algae are placed in the kingdom
A)
Plantae
done
clear
B)
Protista
done
clear
C)
Fungi
done
clear
D)
Monera
done
clear
View Answer play_arrow
question_answer 335) 35. Protozoan, Protista are differentiated on the basis of
A)
Nuclei
done
clear
B)
size
done
clear
C)
shape
done
clear
D)
locomotory structures
done
clear
View Answer play_arrow
question_answer 336) Endoparasitic protistan protozoans belong to
A)
Sporozoa
done
clear
B)
Ciliata
done
clear
C)
Sarcodina
done
clear
D)
Mastigophora
done
clear
View Answer play_arrow
question_answer 337) The winged pollen grains are the characteristic feature of
A)
Cycas
done
clear
B)
Ephedra
done
clear
C)
Gnetum
done
clear
D)
Pinus
done
clear
View Answer play_arrow
question_answer 338) What is the similarity between gymnospenns and angiosperms?
A)
Phloem of both have companian cells
done
clear
B)
Endosperm is formed before fertilization in both
done
clear
C)
Origin of ovule and seed is similar in both
done
clear
D)
Both have leaves, stem and roots
done
clear
View Answer play_arrow
question_answer 339) Circinate venation is found in
A)
Cycas
done
clear
B)
Fern
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 340) In gymnosperm, the endosperm is
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 341) Primitive types of stomata are found in the
A)
leaves of moss plant
done
clear
B)
axis of the moss plant
done
clear
C)
apophysis of capsule of moss
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 342) Paraphysis in moss are
A)
one celled and club shaped
done
clear
B)
multicelled and club shaped
done
clear
C)
one celled and bottle shaped
done
clear
D)
multicelled and bottle shaped
done
clear
View Answer play_arrow
question_answer 343) The development of a sporophyte without fertilization from the vegetative cells of the gametophyte is called
A)
zygospory
done
clear
B)
aplanospory
done
clear
C)
apospory
done
clear
D)
apogamy
done
clear
View Answer play_arrow
question_answer 344) One of the following is not a method of asexual reproduction
A)
cutting
done
clear
B)
grafting
done
clear
C)
budding
done
clear
D)
conjugation
done
clear
View Answer play_arrow
question_answer 345) In Capsella, meiosis takes place during
A)
development of pollen grains
done
clear
B)
development of egg
done
clear
C)
gemination of zygote
done
clear
D)
development of ovule
done
clear
View Answer play_arrow
question_answer 346) Generally archesporium in an ovule is
A)
single celled and hypodermal in origin
done
clear
B)
single celled and lies in the centre of the ovule
done
clear
C)
single celled and terminal in origin
done
clear
D)
many celled and lie in the centre
done
clear
View Answer play_arrow
question_answer 347) When the pollen grains are not transferred from anthers to the stigma in flower due to the barrier of fence, it is referred as or when some natural barriers, exist between androecium and gynoecium to check self pollination, it is known as
A)
heterostyly
done
clear
B)
herkogamy
done
clear
C)
dichogamy
done
clear
D)
cleistogamy
done
clear
View Answer play_arrow
question_answer 348) Aerenchyma is found in
A)
lithophytes
done
clear
B)
hydrophytes
done
clear
C)
sciophytes
done
clear
D)
xerophytes
done
clear
View Answer play_arrow
question_answer 349) Collenchyma differs from sclerenchyma
A)
retaining protoplasm at maturity
done
clear
B)
having thick walls
done
clear
C)
having wide lumen
done
clear
D)
being meristematic
done
clear
View Answer play_arrow
question_answer 350) Water stomata are found in
A)
plants inhabiting humid region
done
clear
B)
plants inhabiting dry regions
done
clear
C)
all plants
done
clear
D)
plants lacking normal stomata
done
clear
View Answer play_arrow
question_answer 351) Multiple epidermis on dorsal and ventral side of the leaf is found in
A)
Zea mays
done
clear
B)
Triticum aestivum
done
clear
C)
Mangifera indica
done
clear
D)
Nerium oleander
done
clear
View Answer play_arrow
question_answer 352) The axillary buds arise
A)
endogenously from the pericycle
done
clear
B)
exogenously from the tissues of the main growing point
done
clear
C)
endogenously from the cambial tissues
done
clear
D)
exogenously from the innermost cortex
done
clear
View Answer play_arrow
question_answer 353) Potassium is useful in development of
A)
fibre
done
clear
B)
pith
done
clear
C)
parenchyma
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 354) Plants absorb sulphur in the form of
A)
\[S{{O}_{4}}\] from soil
done
clear
B)
\[S{{O}_{2}}\] from air
done
clear
C)
Both [a] and [b]
done
clear
D)
\[S{{O}_{3}}\] from soil
done
clear
View Answer play_arrow
question_answer 355) Grey spots of oat are caused by deficiency of
A)
Cu
done
clear
B)
Zn
done
clear
C)
Mn
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 356) Motile leaf in citrus plants is due to deficienp
A)
boron
done
clear
B)
magnesium
done
clear
C)
zinc
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 357) Nodules with nitrogen fixing bacteria present in roots of
A)
cotton
done
clear
B)
gram
done
clear
C)
wheat
done
clear
D)
maize
done
clear
View Answer play_arrow
question_answer 358) A pigment, which absorbed red and far-red light is
A)
phytochrome
done
clear
B)
carotene
done
clear
C)
cytochrome
done
clear
D)
xanthophylls
done
clear
View Answer play_arrow
question_answer 359) 85-90% of all photosynthesis of the world is carried out by
A)
shrubs
done
clear
B)
herbs
done
clear
C)
oceanic algae
done
clear
D)
trees with large branches
done
clear
View Answer play_arrow
question_answer 360) The role of phycobilins in photosynthes is to
A)
absorb and transfer energy to chlorophyll
done
clear
B)
donate electrons to the electron transport system
done
clear
C)
fix carbon dioxide
done
clear
D)
carry hydrogen or electrons
done
clear
View Answer play_arrow
question_answer 361) Chlorophyll-b differs from chlorophyll-a in the it does not
A)
CHO
done
clear
B)
COOH
done
clear
C)
\[C{{H}_{3}}\] and-CH
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 362) Plants do not store carbohydrate as glucose but do so as starch because glucose
A)
is unstable
done
clear
B)
attracts herbivores
done
clear
C)
will change nucleic acids
done
clear
D)
alters osmotic balance
done
clear
View Answer play_arrow
question_answer 363) If oxygen content is reduced to 1%, the rate of respiration becomes
A)
optimum
done
clear
B)
minimum
done
clear
C)
maximum
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 364) Oxidative phosphorylation is the formation of
A)
\[NADP{{H}_{2}}\] in respiration
done
clear
B)
ATP in respiration
done
clear
C)
\[NADP{{H}_{2}}\] in photosynthesis
done
clear
D)
ATP in photosynthesis
done
clear
View Answer play_arrow
question_answer 365) A typical anther wall has
A)
exothecium and endothecium
done
clear
B)
endothecium and tapetum
done
clear
C)
exothecium, endothecium and tapetum
done
clear
D)
exothecium and tapetum
done
clear
View Answer play_arrow
question_answer 366) The tissue of highest respiratory activity is
A)
meristems
done
clear
B)
ground tissue
done
clear
C)
phloem
done
clear
D)
mechanical tissue
done
clear
View Answer play_arrow
question_answer 367) Why do fruit juices turn bitter if kept in an open place for some time?
A)
Juices have something inside them which makes it bitter
done
clear
B)
It is due to fermentation brought about by yeast cells
done
clear
C)
It is due to the activity of fungi present in the atmosphere
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 368) Which one belongs to hydrolase group?
A)
Amylase
done
clear
B)
Transaminase
done
clear
C)
Citrate synthetase
done
clear
D)
Enolase
done
clear
View Answer play_arrow
question_answer 369) Dry seeds can enduce higher temperature that the germinating seeds because
A)
dry seeds have more reserve food
done
clear
B)
hydration makes the enzymes more sensitive to temperature
done
clear
C)
dry seeds are hard
done
clear
D)
the seedlings are tender
done
clear
View Answer play_arrow
question_answer 370) Name of the enzyme that acts both as carboxylase at one time and oxygenase at another time
A)
Carbonic anhydrase
done
clear
B)
PEP carboxylase
done
clear
C)
RuBP carboxylase
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 371) Enzymes, which act normally with in cells are ailed
A)
apoenzymes
done
clear
B)
exoenzymes
done
clear
C)
endoenzymes
done
clear
D)
ferments
done
clear
View Answer play_arrow
question_answer 372) A coenzyme is
A)
same enzyme that occurs in different tissues such as heart and muscle
done
clear
B)
one that shares the function of other enzyme
done
clear
C)
organic or inorganic in nature and helps activate metabolic enzymes
done
clear
D)
organic non-protein in nature and helps to activate metabolic enzymes
done
clear
View Answer play_arrow
question_answer 373) When food supply is poor, the rate of growth is
A)
fast
done
clear
B)
slow
done
clear
C)
intermediate
done
clear
D)
nil
done
clear
View Answer play_arrow
question_answer 374) Energy for the early growth of a developing bean embryo comes from
A)
sunlight
done
clear
B)
water in the soil
done
clear
C)
food in the soil
done
clear
D)
leaves in the seed
done
clear
View Answer play_arrow
question_answer 375) Plant response to environment is mainly through
A)
induction of dormancy
done
clear
B)
abscission of parts
done
clear
C)
synthesis of pigments
done
clear
D)
growth
done
clear
View Answer play_arrow
question_answer 376) Substances, which originate at the tip of stem, root and control the growth of different organs are
A)
enzymes
done
clear
B)
hormones
done
clear
C)
vitamins
done
clear
D)
food substances
done
clear
View Answer play_arrow
question_answer 377) Gibberellins induce
A)
flowering
done
clear
B)
production of hydrolyzing enzymes in germinating seeds
done
clear
C)
Both [a] and [c]
done
clear
D)
hasten leaf senescence
done
clear
View Answer play_arrow
question_answer 378) One of the preventive methods of fruit drop is by spraying
A)
auxin
done
clear
B)
ethylene gas
done
clear
C)
gibberellins
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 379) An important finding in Went's experiment was
A)
unequal distribution of elongation promoting substance in Avena coleoptile
done
clear
B)
presence of elongation factor in all cells of root
done
clear
C)
curvature of coleoptile is proportional to auxin concentration
done
clear
D)
curvature occurred due to irregular elongation of cells
done
clear
View Answer play_arrow
question_answer 380) A bird enters the mouth of crocodile and feed on parasitic leeches. The bird gets food and crocodile gets ribs of blood sucking leeches. Both the partners can live independently. Such an association is
A)
mutualism
done
clear
B)
amensalism
done
clear
C)
commensalism
done
clear
D)
proto co-operation
done
clear
View Answer play_arrow
question_answer 381) Which of the following species are restricted to a given area ?
A)
Sympatric species
done
clear
B)
All opatric species
done
clear
C)
Sibling species
done
clear
D)
Endemic species
done
clear
View Answer play_arrow
question_answer 382) The factor governing the structure of earth surface is
A)
topographic
done
clear
B)
edaphic
done
clear
C)
biotic
done
clear
D)
temperature
done
clear
View Answer play_arrow
question_answer 383) Excessive aerenchyma is characteristic of
A)
hydrophytes
done
clear
B)
xerophytes
done
clear
C)
mesophytes
done
clear
D)
heliophytes
done
clear
View Answer play_arrow
question_answer 384) Species are considered as
A)
real unit of classification devised by taxonomists
done
clear
B)
real basic units of classification
done
clear
C)
the lowest units of classification
done
clear
D)
artificial concept of human mind, which cannot be defined in absolute terms
done
clear
View Answer play_arrow
question_answer 385) Mark the correct pair
A)
plants growing in shady - heliophytes places
done
clear
B)
plants growing in light - sdophytes
done
clear
C)
plants growing in saline - halophytes soil
done
clear
D)
roots are absent - xerophytes
done
clear
View Answer play_arrow
question_answer 386) Ephemerals are xerophytes that are
A)
draught escapers
done
clear
B)
true xerophytes
done
clear
C)
draught resisting
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 387) The sun loving plants are referred to as
A)
halophytes
done
clear
B)
heliophytes
done
clear
C)
heterotrophs
done
clear
D)
heliophytes
done
clear
View Answer play_arrow
question_answer 388) The lentic ecosystem includes, which of the following water?
A)
Rain
done
clear
B)
Running
done
clear
C)
Standing
done
clear
D)
Gravitational
done
clear
View Answer play_arrow
question_answer 389) Succulent xerophytes are likely to be found in
A)
tropical rain forest
done
clear
B)
deciduous forest
done
clear
C)
desert
done
clear
D)
tundra
done
clear
View Answer play_arrow
question_answer 390) Which alkaloids are present in opium ?
A)
Codeine
done
clear
B)
Morphine
done
clear
C)
Perthidine
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 391) Which of the following is used as local an aesthesia?
A)
Aloe
done
clear
B)
Cocaine
done
clear
C)
Calotropis latex
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 392) Study of drug plants is known as
A)
Pharmacy
done
clear
B)
Pharmacology
done
clear
C)
Pharmacognosy
done
clear
D)
Pharmaceutical chemistry
done
clear
View Answer play_arrow
question_answer 393) The correct representation of the alkaloids obtained from opium is
A)
Morphine > Codeine > Pethidine
done
clear
B)
Morphine < Codeine < Perthidine
done
clear
C)
Morphine > Codeine > Perthidine
done
clear
D)
Morphine < Codeine < Nicotine
done
clear
View Answer play_arrow
question_answer 394) Cinchona plant is also known as
A)
prickly bark
done
clear
B)
turmeric bark
done
clear
C)
peruvian bark
done
clear
D)
devil's dung bark
done
clear
View Answer play_arrow
question_answer 395) Which is more suitable in jelly preparation?
A)
Sugar
done
clear
B)
Salt
done
clear
C)
Pectin
done
clear
D)
Protein
done
clear
View Answer play_arrow
question_answer 396) Botulism is a
A)
type of food poisoning due to saprophya Clostridium botulinum bacterium
done
clear
B)
disease of man due to parasitic bacterium
done
clear
C)
disease in various organisms
done
clear
D)
disease of plants due to viruses
done
clear
View Answer play_arrow
question_answer 397) In fruit preservation, which substance is used as preservative?
A)
Calcium carbonate
done
clear
B)
Potassium metabisulphate
done
clear
C)
Potassium nitrate
done
clear
D)
Ammonium sulphate
done
clear
View Answer play_arrow
question_answer 398) The main aim of plant breeding is
A)
to produce improved varieties
done
clear
B)
to make soil fertile
done
clear
C)
to control pollution
done
clear
D)
to become more progressive
done
clear
View Answer play_arrow
question_answer 399) Which cultivation method is most popular in Madhya Pradesh to cultivate rice?
A)
Intensive
done
clear
B)
Dry
done
clear
C)
Wet
done
clear
D)
Tillage
done
clear
View Answer play_arrow
question_answer 400) Dwarf wheats were developed by
A)
Vavilov
done
clear
B)
Borlaug
done
clear
C)
Swaminathan
done
clear
D)
None of the above
done
clear
View Answer play_arrow