A) \[NO_{3}^{-},CO_{3}^{2-}\]
B) \[S{{O}_{3}},NO_{3}^{-}\]
C) \[ClO_{3}^{-},CO_{3}^{2-}\]
D) \[CO_{3}^{2-},S{{O}_{3}}\]
Correct Answer: A
Solution :
Isoelectronic means same number of electrons and isostructural means same structure. \[NO_{3\,\,}^{-}\,\,\,\,\,\,\,\,\,7+8\times 3+1=32\] \[CO_{3}^{2-}\,\,\,\,\,\,\,\,\,6+8\times 3+2=32\]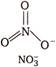
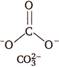 Hence, \[NO_{3}^{-}\] and \[CO_{3}^{2-}\] are isoelectronic and isostructural both.
Hence, \[NO_{3}^{-}\] and \[CO_{3}^{2-}\] are isoelectronic and isostructural both.
You need to login to perform this action.
You will be redirected in
3 sec
