A) \[MO\]
B) \[{{M}_{2}}O\]
C) \[{{M}_{2}}{{O}_{3}}\]
D) \[M{{O}_{2}}\]
Correct Answer: B
Solution :
The electronic configuration of a metal (M) is \[=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{1}},\]so valency of metal (M) is 1. Valency of oxygen \[=2\]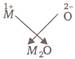 or
or 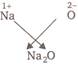 \[\therefore \] The formula of metal oxide is \[{{M}_{2}}O\].
\[\therefore \] The formula of metal oxide is \[{{M}_{2}}O\].
You need to login to perform this action.
You will be redirected in
3 sec
