question_answer 1) A proton beam enters a magnetic field of \[{{10}^{-4}}\text{ }Wb\text{ }{{m}^{-2}}\]1normally. If the specific charge of the proton is \[{{10}^{11}}C\text{ }k{{g}^{-1}}\]and its velocity is \[{{10}^{9}}m\,{{s}^{-1}}\], then the radius of the circle described will be
A)
\[10m\]
done
clear
B)
\[1\text{ }m~\]
done
clear
C)
\[0.1m\]
done
clear
D)
\[100m\]
done
clear
View Answer play_arrow
question_answer 2) Two concentric coils each of radius equal to \[2\pi cm\]are placed right angles to each other. If 3 A and 4 A are the currents flowing through the two coils respectively. The magnetic induction (in Wb\[{{m}^{-2}}\]) at the centre of the coils will be
A)
\[{{10}^{-5}}\]
done
clear
B)
\[7\times {{10}^{-5}}\]
done
clear
C)
\[12\times {{10}^{-5}}\]
done
clear
D)
\[5\times {{10}^{-5}}\]
done
clear
View Answer play_arrow
question_answer 3) The resistance of the bulb filament is \[100\Omega \] at a temperature of \[{{100}^{{}^\circ }}C\]. If its temperature coefficient of resistance be 0.005 per \[^{{}^\circ }C\], its resistance will become \[200\Omega \] at a temperature
A)
\[{{400}^{{}^\circ }}C\]
done
clear
B)
\[{{200}^{{}^\circ }}C\]
done
clear
C)
\[{{300}^{{}^\circ }}C\]
done
clear
D)
\[{{500}^{{}^\circ }}C\]
done
clear
View Answer play_arrow
question_answer 4) In Wheatstones network \[P=2\Omega ,\,Q=2\Omega ,\,R=2\Omega \]and\[S=3\Omega \]. The resistance with which S is to shunted in order that the bridge may be balanced is
A)
\[2\Omega \]
done
clear
B)
\[6\Omega \]
done
clear
C)
\[1\Omega \]
done
clear
D)
\[4\Omega \]
done
clear
View Answer play_arrow
question_answer 5) Core of electromagnets are made of ferromagnetic material which has
A)
high permeability and high retentivity
done
clear
B)
low permeability and low retentivity
done
clear
C)
high permeability and low retentivity
done
clear
D)
low permeability and high retentivity
done
clear
View Answer play_arrow
question_answer 6) If there is no torsion in the suspension thread, then the time period of a magnet executing SHM is
A)
\[T=\frac{1}{2\pi }\sqrt{\frac{I}{MB}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{MB}{I}}\]
done
clear
C)
\[T=\frac{1}{2\pi }\sqrt{\frac{MB}{I}}\]
done
clear
D)
\[T=2\pi \sqrt{\frac{I}{MB}}\]
done
clear
View Answer play_arrow
question_answer 7) Two parallel wires 1 m apart carry currents of 1 A and 3 A respectively in opposite directions. The force per unit length acting between these two wires is
A)
\[6\times {{10}^{-7}}N\text{ }{{m}^{-1}}\text{ }attractive\]
done
clear
B)
\[6\times {{10}^{-5}}\text{ }N\text{ }{{m}^{-1}}\text{ }attractive\]
done
clear
C)
\[6\times {{10}^{-7}}\text{ }N\text{ }{{m}^{-1}}\text{ }repulsive\]
done
clear
D)
\[6\times {{10}^{-5}}\text{ }N\text{ }{{m}^{-1}}\text{ }repulsive\]
done
clear
View Answer play_arrow
question_answer 8) A galvanometer of resistance \[50\Omega \] gives a full scale deflection for a current \[5\times {{10}^{-4}}A\]. The resistance that should be connected in series with the galvanometer to read 3 V is
A)
\[5050\Omega \]
done
clear
B)
\[5950\Omega \]
done
clear
C)
\[595\Omega \]
done
clear
D)
\[5059\Omega \]
done
clear
View Answer play_arrow
question_answer 9) A cyclotron is used to accelerate
A)
only positively charged particles
done
clear
B)
both positively and negatively charged particles
done
clear
C)
Neutron
done
clear
D)
Only negatively charged particles
done
clear
View Answer play_arrow
question_answer 10) A transformer is used to light 100 W-110 V lamp from 220 V mains. If the main current is 0.5 A, the efficiency of the transformer is
A)
\[95%\]
done
clear
B)
\[99%\]
done
clear
C)
\[90%\]
done
clear
D)
\[96%\]
done
clear
View Answer play_arrow
question_answer 11) In an LCR circuit, at resonance
A)
The impedance is maximum
done
clear
B)
The current leads the voltage by \[\pi /2\]
done
clear
C)
The current and voltage are in phase
done
clear
D)
The current is minimum
done
clear
View Answer play_arrow
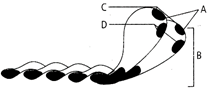
question_answer 12) An aircraft with a wingspan of 40 m flies with a speed of 1080 km/hr in the eastward direction at a constant altitude in the northern hemisphere, where the vertical component of the earths magnetic field \[1.75\times {{10}^{-5}}T\]. Then the emf developed between the tips of the wings is
A)
\[0.34V\]
done
clear
B)
\[2.1V\]
done
clear
C)
\[0.5V\]
done
clear
D)
\[0.21V\]
done
clear
View Answer play_arrow
question_answer 13) Two coils have a mutual inductance 0.005 H. The current changes in the first coil according to the equation \[i={{i}_{m}}\sin \omega t\] where \[{{i}_{m}}=10A\] and \[\omega =100\pi \,\,rad\,\,{{s}^{-1}}\]. The maximum value of the emf induced in the second coil is
A)
\[5\pi \]
done
clear
B)
\[4\pi \]
done
clear
C)
\[2\pi \]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 14) The magnetic susceptibility of a paramagnetic material at \[-{{73}^{o}}C\]is 0.0075 and its value at \[-{{173}^{o}}C\]will be
A)
\[0.0030\]
done
clear
B)
\[0.0075\]
done
clear
C)
\[0.0045~\]
done
clear
D)
\[0.015\]
done
clear
View Answer play_arrow
question_answer 15) In a Youngs double slit experiment the slit separation is 0.5 m from the slits. For a monochromatic light of wavelength 500 nm, the distance of 3rd maxima from 2nd minima on the other side is
A)
\[2.5mm\]
done
clear
B)
\[2.25mm\]
done
clear
C)
\[2.75mm\]
done
clear
D)
\[22.5mm\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 16) Calculate the focal length of a reading glass of a person if his distance of distinct vision is 75 cm.
A)
\[37.5cm\]
done
clear
B)
\[100.4cm\]
done
clear
C)
\[25.6cm\]
done
clear
D)
\[75.2cm\]
done
clear
View Answer play_arrow
question_answer 17) A person wants a real image of his own, 3 times enlarged. Where should he stand in front of a concave mirror of radius of curvature 30 cm?
A)
\[30cm\]
done
clear
B)
\[20cm\]
done
clear
C)
\[10cm\]
done
clear
D)
\[90cm\]
done
clear
View Answer play_arrow
question_answer 18) If \[{{\varepsilon }_{0}}\] and \[{{\mu }_{0}}\] are the permittivity and permeability of free space and e and \[\mu \] are the corresponding quantities for a medium, then refractive index of the medium is
A)
\[\sqrt{\frac{\mu \varepsilon }{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
B)
Insufficient information
done
clear
C)
\[\sqrt{\frac{{{\mu }_{0}}{{\varepsilon }_{0}}}{\mu \varepsilon }}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 19) The average power dissipated in a pure inductor is
A)
\[V{{I}^{2}}\]
done
clear
B)
zero
done
clear
C)
\[\frac{1}{2}VI\]
done
clear
D)
\[\frac{V{{I}^{2}}}{4}\]
done
clear
View Answer play_arrow
question_answer 20) An a-particle of energy 5 MeV is scattered through \[{{180}^{o}}\] by gold nucleus. The distance of closest approach is of the order of
A)
\[{{10}^{-12}}cm\]
done
clear
B)
\[{{10}^{-16}}cm\]
done
clear
C)
\[{{10}^{-10}}cm\]
done
clear
D)
\[{{10}^{-14}}cm\]
done
clear
View Answer play_arrow
question_answer 21) Find the de-Broglie wavelength of an electron with kinetic energy of 120 eV.
A)
\[102pm\]
done
clear
B)
\[124pm\]
done
clear
C)
\[95pm\]
done
clear
D)
\[112pm\]
done
clear
View Answer play_arrow
question_answer 22) Light of two different frequencies whose photons have energies 1 eV and 2.5 eV respectively, successively illuminate a metallic surface whose work function is 0.5 eV. Ratio of maximum speeds of emitted electrons will be
A)
\[1:4\]
done
clear
B)
\[1:1\]
done
clear
C)
\[1:5\]
done
clear
D)
\[1:2\]
done
clear
View Answer play_arrow
question_answer 23) The polarizing angle of glass is \[{{57}^{o}}\]. A ray of light which is incident at this angle will have an angle of refraction as
A)
\[{{33}^{o}}\]
done
clear
B)
\[{{38}^{o}}\]
done
clear
C)
\[{{25}^{o}}\]
done
clear
D)
\[{{43}^{o}}\]
done
clear
View Answer play_arrow
question_answer 24) To observe diffraction, the size of the obstacle
A)
Should be \[\lambda /2\], where \[\lambda \] is the wavelength.
done
clear
B)
Should be of the order of wavelength.
done
clear
C)
Has no relation to wavelength.
done
clear
D)
should be much larger than the wavelength.
done
clear
View Answer play_arrow
question_answer 25) A radioactive decay can form an isotope of the original nucleus with the emission of particles
A)
One \[\alpha \] and two \[\beta \]
done
clear
B)
Four \[\alpha \] and one \[\beta \]
done
clear
C)
One \[\alpha \]and four \[\beta \]
done
clear
D)
One \[\alpha \] and one \[\beta \]
done
clear
View Answer play_arrow
question_answer 26) The half-life of a radioactive substance is 20 minutes. The time taken between 50% decay and 87.5% decay of the substance will be
A)
40 minutes
done
clear
B)
10 minutes
done
clear
C)
30 minutes
done
clear
D)
25 minutes
done
clear
View Answer play_arrow
question_answer 27) A nucleus at rest splits into two nuclear parts having radii in the ratio\[1:2\]. Their velocities are in the ratio
A)
\[6:1\]
done
clear
B)
\[2:1\]
done
clear
C)
\[8:1\]
done
clear
D)
\[4:1\]
done
clear
View Answer play_arrow
question_answer 28) What is the wavelength of light for the least energetic photon emitted in the Lyman series of the hydrogen spectrum? (Take he = 1240 eV nm)
A)
\[102nm\]
done
clear
B)
\[150nm\]
done
clear
C)
\[82nm\]
done
clear
D)
\[122nm\]
done
clear
View Answer play_arrow
question_answer 29) If an electron in hydrogen atom jumps from an orbit of level n = 3 to an orbit of level n = 2, the emitted radiation has a frequency (R = Rydberg constant, C = velocity of light)
A)
\[\frac{RC}{25}\]
done
clear
B)
\[\frac{5RC}{36}\]
done
clear
C)
\[\frac{3RC}{27}\]
done
clear
D)
\[\frac{8RC}{9}\]
done
clear
View Answer play_arrow
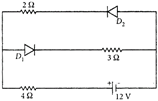
question_answer 30)
The circuit has two oppositely connected ideal diodes in parallel. What is the current flowing in the circuit?
A)
\[2.0A\]
done
clear
B)
\[1.33A\]
done
clear
C)
\[1.71\text{ }A\]
done
clear
D)
\[2.31A\]
done
clear
View Answer play_arrow
question_answer 31) Amplitude modulation has
A)
One carrier
done
clear
B)
One carrier with high frequency
done
clear
C)
One carrier with two side band frequencies
done
clear
D)
One carrier with infinite frequencies
done
clear
View Answer play_arrow
question_answer 32) An LED is constructed from a pn junction based on a certain semi-conducting material whose energy gap is 1.9 eV. Then the wavelength of the emitted light is
A)
\[1.6\times {{10}^{-8}}m\]
done
clear
B)
\[9.1\times {{10}^{-5}}m\]
done
clear
C)
\[2.9\times {{10}^{-9}}m\]
done
clear
D)
\[6.5\times {{10}^{-7}}m\]
done
clear
View Answer play_arrow
question_answer 33) The waves used for line-of-sight (LOS) communication is
A)
Space waves
done
clear
B)
Sky waves
done
clear
C)
Ground waves
done
clear
D)
Sound waves
done
clear
View Answer play_arrow
question_answer 34)
The given truth table is for Input Out Put A B Y 0 0 1 0 1 1 1 0 1 1 1 0
A)
OR gate
done
clear
B)
NOR gate
done
clear
C)
AND gate
done
clear
D)
NAND gate
done
clear
View Answer play_arrow
question_answer 35) The input characteristics of a transistor in CE mode is the graph obtained by plotting
A)
\[{{I}_{B}}\] against \[{{V}_{CE}}\] at constant \[{{V}_{BE}}\]
done
clear
B)
\[{{I}_{B}}\] against \[{{I}_{C}}\] at constant \[{{V}_{BE}}\]
done
clear
C)
\[{{I}_{B}}\] against \[{{V}_{BE}}\] at constant \[{{V}_{CE}}\]
done
clear
D)
\[{{I}_{B}}\] against \[{{I}_{C}}\] at constant \[{{V}_{CE}}\]
done
clear
View Answer play_arrow
question_answer 36) A particle is projected with a velocity v so that its horizontal range twice the greatest height attained. The horizontal range is
A)
\[\frac{2{{V}^{2}}}{3g}\]
done
clear
B)
\[\frac{{{v}^{2}}}{2g}\]
done
clear
C)
\[\frac{{{v}^{2}}}{g}\]
done
clear
D)
\[\frac{4{{v}^{2}}}{5g}\]
done
clear
View Answer play_arrow
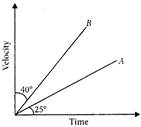
question_answer 37)
The velocity - time graph for two bodies A and B are shown. Then the acceleration of A and B are in the ratio
A)
\[tan{{25}^{o}}\]to \[tan{{50}^{o}}\]
done
clear
B)
\[cos{{25}^{o}}\]to \[cos{{50}^{o}}\]
done
clear
C)
\[\tan {{25}^{o}}\] to \[\tan {{40}^{o}}\]
done
clear
D)
\[\sin {{25}^{o}}\] to \[\sin {{50}^{o}}\]
done
clear
View Answer play_arrow
question_answer 38) The ratio of the dimensions of Planck constant and that of moment of inertia has the dimensions of
A)
Frequency
done
clear
B)
Velocity
done
clear
C)
Time
done
clear
D)
Angular momentum
done
clear
View Answer play_arrow
question_answer 39) Moment of inertia of a thin uniform rod rotating about the perpendicular axis passing through its centre is I. If the same rod is bent into a ring and its moment of inertia about its diameter is I, then the ratio \[\frac{I}{I}\] is
A)
\[\frac{8}{3}{{\pi }^{2}}\]
done
clear
B)
\[\frac{5}{3}{{\pi }^{2}}\]
done
clear
C)
\[\frac{3}{2}{{\pi }^{2}}\]
done
clear
D)
\[\frac{2}{3}{{\pi }^{2}}\]
done
clear
View Answer play_arrow
question_answer 40) If the mass of a body is M on the surface of the earth, the mass of the same body on the surface of the moon is
A)
\[M\]
done
clear
B)
zero
done
clear
C)
\[\frac{M}{6}\]
done
clear
D)
\[6M\]
done
clear
View Answer play_arrow
question_answer 41) The ratio of angular speed of a second-hand to the hour-hand of a watch is
A)
\[60:1\]
done
clear
B)
\[72:1\]
done
clear
C)
\[720:1\]
done
clear
D)
\[3600:1\]
done
clear
View Answer play_arrow
question_answer 42) The kinetic energy of a body of mass 4 kg and momentum 6 N s will be
A)
\[3.5J~\]
done
clear
B)
\[5.5J~\]
done
clear
C)
\[2.5J~\]
done
clear
D)
\[4.5J~\]
done
clear
View Answer play_arrow
question_answer 43) A stone of mass 0.05 kg is thrown vertically upwards. What is the direction and magnitude of net force on the stone during its upward motion?
A)
0.49 N vertically downwards
done
clear
B)
9.8 N vertically downwards
done
clear
C)
0.49 N vertically upwards
done
clear
D)
0.98 N vertically downwards
done
clear
View Answer play_arrow
question_answer 44) The ratio of kinetic energy to the potential energy of a particle executing SHM at a distance equal to half its amplitude, the distance being measured from its equilibrium position is
A)
\[4:1\]
done
clear
B)
\[8:1\]
done
clear
C)
\[3:1\]
done
clear
D)
\[2:1\]
done
clear
View Answer play_arrow
question_answer 45) 1 gram of ice is mixed with 1 gram of steam. At thermal equilibrium, the temperature of the mixture is
A)
\[{{100}^{o}}C\]
done
clear
B)
\[{{55}^{o}}C\]
done
clear
C)
\[{{0}^{o}}C\]
done
clear
D)
\[{{50}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 46) Water is heated from \[{{0}^{o}}C\] to \[{{10}^{o}}C\], then its volume
A)
Increases
done
clear
B)
First decreases and then increases
done
clear
C)
Decreases
done
clear
D)
Does not change
done
clear
View Answer play_arrow
question_answer 47) The efficiency of a Carnot engine which operates between the two temperatures \[{{T}_{1}}=500K\] and \[{{T}_{2}}=300K\] is
A)
\[25%\]
done
clear
B)
\[40%\]
done
clear
C)
\[50%\]
done
clear
D)
\[75%\]
done
clear
View Answer play_arrow
question_answer 48) The ratio of hydraulic stress to the corresponding strain is known as
A)
Bulk modulus
done
clear
B)
Rigidity modulus
done
clear
C)
Compressibility
done
clear
D)
Youngs modulus
done
clear
View Answer play_arrow
question_answer 49) The angle between the dipole moment and electric field at any point on the equatorial plane is
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{0}^{o}}\]
done
clear
D)
\[{{180}^{o}}\]
done
clear
View Answer play_arrow
question_answer 50) Pick out the statement which is incorrect.
A)
The electric field lines forms closed loop.
done
clear
B)
Field lines never intersect.
done
clear
C)
The tangent drawn to a line of force represents the direction of electric field.
done
clear
D)
A negative test charge experiences a force opposite to the direction of the field.
done
clear
View Answer play_arrow
question_answer 51) Two spheres carrying charges \[+6\mu C\] and \[+9\mu C\], separated by a distance d, experiences a force of repulsion F. When a charge of \[-3\mu C\]is given to both the sphere and kept at the same distance as before, the new force of repulsion is
A)
\[3F\]
done
clear
B)
\[\frac{F}{9}\]
done
clear
C)
\[F\]
done
clear
D)
\[\frac{F}{3}\]
done
clear
View Answer play_arrow
question_answer 52) A stretched string is vibrating in the second overtone, then the number of nodes and antinodes between the ends of the string are respectively
A)
3 and 2
done
clear
B)
2 and 3
done
clear
C)
4 and 3
done
clear
D)
3 and 4
done
clear
View Answer play_arrow
question_answer 53) When two tuning forks A and B are sounded together, 4 beats per second are heard. The frequency of the fork B is 384 Hz. When one of the prongs of the fork A is filed and sounded with B, the beat frequency increases, then the frequency of the fork A is
A)
\[388Hz\]
done
clear
B)
\[389Hz\]
done
clear
C)
\[380Hz\]
done
clear
D)
\[379Hz\]
done
clear
View Answer play_arrow
question_answer 54) Three resistances \[2\Omega \], \[3\Omega \]. and \[4\Omega \] are connected in parallel. The ratio of currents passing through them when a potential difference is applied across its ends will be
A)
\[6:4:3\]
done
clear
B)
\[4:3:2\]
done
clear
C)
\[6:3:2\]
done
clear
D)
\[5:4:3\]
done
clear
View Answer play_arrow
question_answer 55) Four identical cells of emf E and internal resistance r are to be connected in series. Suppose if one of the cell is connected wrongly, the equivalent emf and effective internal resistance of the combination is
A)
\[4E\]and \[2r\]
done
clear
B)
\[2E\] and \[2r\]
done
clear
C)
\[4E\] and \[4r\]
done
clear
D)
\[2E\] and \[4r\]
done
clear
View Answer play_arrow
question_answer 56) A parallel plate capacitor is charged and then isolated. The effect of increasing the plate separation on charge, potential and capacitance respectively are
A)
Increases, decreases, decreases
done
clear
B)
Constant, increases, decreases
done
clear
C)
Constant, decreases, decreases
done
clear
D)
Constant, decreases, increases
done
clear
View Answer play_arrow
question_answer 57) A spherical shell of radius 10 cm is carrying a charge q. If the electric potential at distances .5 cm, 10 cm and 15 cm from the center of the spherical shell is Vi, V. and V^ respectively, then
A)
\[{{V}_{1}}<{{V}_{2}}<{{V}_{3}}\]
done
clear
B)
\[{{V}_{1}}={{V}_{2}}<{{V}_{3}}\]
done
clear
C)
\[{{V}_{1}}>{{V}_{2}}>{{V}_{3}}\]
done
clear
D)
\[{{V}_{1}}={{V}_{2}}>{{V}_{3}}\]
done
clear
View Answer play_arrow
question_answer 58) Three point charges \[3\,nC\], \[6\,nC\] and \[9\,nC\] are placed at the corners of an equilateral triangle of side 0.1m. The potential energy of the system is
A)
\[89100J\]
done
clear
B)
\[99100J\]
done
clear
C)
\[8910\text{ }J\]
done
clear
D)
\[9910J\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
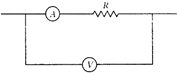
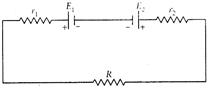
question_answer 59)
In the circuit shown below, the ammeter and the voltmeter readings are 3 A and 6 V respectively. Then the value of the resistance R is
A)
\[>2\Omega \]
done
clear
B)
\[\ge 2\Omega \]
done
clear
C)
\[2\Omega \]
done
clear
D)
\[<2\Omega \]
done
clear
View Answer play_arrow
question_answer 60)
Two cells of emf \[{{E}_{1}}\] and \[{{E}_{2}}\] are joined in opposition (such that \[{{E}_{1}}>{{E}_{2}}\]). If \[{{r}_{1}}\] and \[{{r}_{2}}\] be the internal resistance and R be the external resistance, then the terminal potential difference is
A)
\[\frac{{{E}_{1}}+{{E}_{2}}}{{{r}_{1}}+{{r}_{2}}+R}\times R\]
done
clear
B)
\[\frac{{{E}_{1}}-{{E}_{2}}}{{{r}_{1}}+{{r}_{2}}+R}\times R\]
done
clear
C)
\[\frac{{{E}_{1}}+{{E}_{2}}}{{{r}_{1}}+{{r}_{2}}}\times R\]
done
clear
D)
\[\frac{{{E}_{1}}-{{E}_{2}}}{{{r}_{1}}+{{r}_{2}}}\times R\]
done
clear
View Answer play_arrow
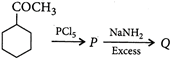
question_answer 61) \[0.30g\]of an organic compound containing C, H and O on combustion yields \[0.44g\,C{{O}_{2}}\]and\[0.18g\,{{H}_{2}}O\]. If one mole of compound weighs 60, then molecular formula of the compound is
A)
\[{{C}_{3}}{{H}_{8}}O\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
C)
\[C{{H}_{2}}O\]
done
clear
D)
\[{{C}_{4}}{{H}_{6}}O\]
done
clear
View Answer play_arrow
question_answer 62)
For one of the element various successive ionization enthalpies (in\[kJ\text{ }mo{{l}^{-1}}\]) are given below: I.E 1st 2nd 3rd 4th 5th \[577.5\] \[1810\] \[2750\] \[11,580\] \[14,820\]
The element is
A)
\[P\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Si\]
done
clear
D)
\[Al\]
done
clear
View Answer play_arrow
question_answer 63) The aqueous solution of following salt will have the lowest \[pH\]
A)
\[NaClO\]
done
clear
B)
\[NaCl{{O}_{4}}\]
done
clear
C)
\[NaCl{{O}_{3}}\]
done
clear
D)
\[NaCl{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) One of the following is an essential amino acid.
A)
Cysteine
done
clear
B)
Serine
done
clear
C)
Tyrosine
done
clear
D)
Isoleucine
done
clear
View Answer play_arrow
question_answer 65) The formation of cyanohydrin from a ketone is an example of
A)
nucleophilic addition
done
clear
B)
electrophilic substitution
done
clear
C)
nucleophilic substitution
done
clear
D)
electrophilic addition.
done
clear
View Answer play_arrow
question_answer 66) \[100c{{m}^{3}}\]of \[1M\text{ }C{{H}_{3}}COOH\]was mixed with \[100\text{ }c{{m}^{3}}\]of \[2M\text{ }C{{H}_{3}}OH\]to form an ester. The change in the initial rate if each solution is diluted with equal volume of water would be
A)
4 times
done
clear
B)
0.25 times
done
clear
C)
2 times
done
clear
D)
0.5 times.
done
clear
View Answer play_arrow
question_answer 67) How many coulombs of electricity are required for the oxidation of one mol of water to dioxygen?
A)
\[1.93\times {{10}^{4}}C\]
done
clear
B)
\[19.3\times {{10}^{5}}C\]
done
clear
C)
\[9.65\times {{10}^{4}}C\]
done
clear
D)
\[1.93\times {{10}^{5}}C\]
done
clear
View Answer play_arrow
question_answer 68) Cheilosis and digestive disorders are due to the deficiency of
A)
ascorbic acid
done
clear
B)
pyridoxine
done
clear
C)
thiamine
done
clear
D)
riboflavin.
done
clear
View Answer play_arrow
question_answer 69) One of the following amides will not undergo Hoffmann bromamide reaction:
A)
\[C{{H}_{3}}CONHC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 70) lodoform can be prepared from all, except
A)
butan-2-one
done
clear
B)
acetophenone
done
clear
C)
propan-2-ol
done
clear
D)
propan-1-ol.
done
clear
View Answer play_arrow
question_answer 71)
The arrangement of following compounds : (i) bromomethane (ii) bromoform (iii) chloromethane (iv) dibromomethane
In the increasing order of their boiling point is
A)
\[iv<iii<i<ii\]
done
clear
B)
\[i<ii<iii<iv\]
done
clear
C)
\[iii<i<iv<ii\]
done
clear
D)
\[ii<iii<i<iv\]
done
clear
View Answer play_arrow
question_answer 72) The complex ion having minimum magnitude of \[{{\Delta }_{\upsilon }}(CFSE)\] is
A)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
B)
\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[CoC{{l}_{6}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 73) Which of the following colloids cannot be easily coagulated?
A)
Multimolecular colloids
done
clear
B)
Irreversible colloids
done
clear
C)
Lyophobic colloids
done
clear
D)
Macromolecular colloids
done
clear
View Answer play_arrow
question_answer 74) After adding non-volatile solute freezing point of water decreases to\[-{{0.186}^{o}}C\]. Calculate \[\Delta {{T}_{b}}\], if \[{{K}_{f}}\text{=}1.86\text{ }K\text{ }kg\text{ }mo{{l}^{-1}}\]and \[{{K}_{f}}\text{=}1.86\text{ }K\text{ }kg\text{ }mo{{l}^{-1}}\]
A)
\[0.0521K\]
done
clear
B)
\[0.0186K\]
done
clear
C)
\[0.521K\]
done
clear
D)
\[1.86K\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following compounds of xenon has pyramidal geometry?
A)
\[Xe{{F}_{2}}\]
done
clear
B)
\[Xe{{F}_{4}}\]
done
clear
C)
\[XeO{{F}_{4}}\]
done
clear
D)
\[Xe{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 76) Cryolite is
A)
\[N{{a}_{3}}Al{{F}_{6}}\]and is used in the electrolysis of alumina for lowering the melting point of alumina only
done
clear
B)
\[N{{a}_{3}}Al{{F}_{6}}\] and is used in the electrolytic refining of alumina
done
clear
C)
\[N{{a}_{3}}Al{{F}_{6}}\] and is used in the electrolysis of alumina for decreasing electrical conductivity
done
clear
D)
\[N{{a}_{3}}Al{{F}_{6}}\] and is used in the electrolysis of alumina for lowering the melting point and increasing the conductivity of alumina.
done
clear
View Answer play_arrow
question_answer 77)
Identify Q in the following sequence of reactions:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 78) What amount of dioxygen (in gram) contains \[1.8\times {{10}^{22}}\]molecules?
A)
\[0.960\]
done
clear
B)
\[96.0\]
done
clear
C)
\[0.0960\]
done
clear
D)
\[9.60\]
done
clear
View Answer play_arrow
question_answer 79) The pair of compound which cannot exist together in solution is
A)
\[NaHC{{O}_{3}}\]and \[{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]and \[NaOH\]
done
clear
C)
\[NHC{{O}_{3}}\]and \[NaOH\]
done
clear
D)
\[NaHC{{O}_{3}}\]and \[N{{a}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 80)
Plot of Maxwells distribution of velocities is given below:
A)
\[{{f}_{1}}>{{f}_{2}}\]
done
clear
B)
\[{{V}_{1}}<{{V}_{2}}\]
done
clear
C)
\[{{T}_{1}}<{{T}_{2}}\]
done
clear
D)
\[{{T}_{1}}>{{T}_{2}}\]
done
clear
View Answer play_arrow
question_answer 81) Arrange the following compounds in the increasing order of their acidic strength: (i) m-Nitrophenol (ii) m-Cresol (iii) Phenol (iv) m-Chlorophenol
A)
\[ii<iv<iii<i\]
done
clear
B)
\[ii<iii<i<iv\]
done
clear
C)
\[iii<ii<i<iv\]
done
clear
D)
\[ii<iii<iv<i\]
done
clear
View Answer play_arrow
question_answer 82) . In the reaction: \[S+\frac{3}{2}{{O}_{2}}\xrightarrow{{}}S{{O}_{3}}+2x\,kJ\] and \[S{{O}_{2}}+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}S{{O}_{3}}+y\,kJ\]heat of formation of \[S{{O}_{2}}\] is
A)
\[x-y\]
done
clear
B)
\[2x+y\]
done
clear
C)
\[x+y\]
done
clear
D)
\[2x-y\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 83) Which of the following is not true?
A)
Ampicillin is not a natural antibiotic.
done
clear
B)
Vancomycin is a broad spectrum antibiotic.
done
clear
C)
Erythromycin is a bacteriostatic antibiotic.
done
clear
D)
Prontosil is not converted into sulphanilamide in the body.
done
clear
View Answer play_arrow
question_answer 84) Using MOT, compare \[O_{2}^{+}\] and \[O_{2}^{-}\] species and choose the incorrect option.
A)
\[O_{2}^{-}\]is less stable.
done
clear
B)
Both \[O_{2}^{+}\]and \[O_{2}^{-}\] are paramagnetic.
done
clear
C)
\[O_{2}^{+}\] have higher bond order than\[O_{2}^{-}\] .
done
clear
D)
\[O_{2}^{+}\] is diamagnetic while \[O_{2}^{-}\] is paramagnetic.
done
clear
View Answer play_arrow
question_answer 85) Which of the following compounds possesses the \[C-H\] bond with the lowest bond dissociation energy?
A)
Benzene
done
clear
B)
2,2-Dimethylpropane
done
clear
C)
Toluene
done
clear
D)
n-Pentane
done
clear
View Answer play_arrow
question_answer 86) The correct statement is
A)
\[B{{I}_{3}}\] is the weakest Lewis acid among the boron halides
done
clear
B)
there is minimum \[p\pi -p\pi \] back bonding in \[B{{F}_{3}}\]
done
clear
C)
\[B{{F}_{3}}\] is the strongest Lewis acid among the other boron halides
done
clear
D)
there is maximum \[p\pi -p\pi \] back bonding in \[B{{F}_{3}}\].
done
clear
View Answer play_arrow
question_answer 87) Acetic acid is treated with \[Ca{{(OH)}_{2}}\]and the product so obtained is subjected to dry distillation. The final product is
A)
propanal
done
clear
B)
ethanol
done
clear
C)
ethanal
done
clear
D)
propanone.
done
clear
View Answer play_arrow
question_answer 88)
In the sequence of following reactions:
A)
m-nitro toluene
done
clear
B)
p-nitro toluene
done
clear
C)
p-nitro toluene
done
clear
D)
o-bromotoluene.
done
clear
View Answer play_arrow
question_answer 89) An alkali metal hydride \[(NaH)\] reacts with diborane in A to give a tetrahedral compound B which is extensively used as reducing agent in organic synthesis. The compound A and B respectively are
A)
\[C{{H}_{3}}COC{{H}_{3}}\]and \[{{B}_{3}}{{N}_{3}}{{H}_{6}}\]
done
clear
B)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}O\] and \[NaB{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{6}}\]and \[{{C}_{2}}{{H}_{5}}Na\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}\]and \[NaB{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 90) Water softening by Clarks process uses
A)
\[NaHC{{O}_{3}}\]
done
clear
B)
\[Ca{{(OH)}_{2}}\]
done
clear
C)
\[Ca{{(HC{{O}_{3}})}_{2}}\]
done
clear
D)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 91) One of the following conversion results in the change of hybridization and geometry.
A)
\[N{{H}_{3}}\]to \[NH_{4}^{+}\]
done
clear
B)
\[{{H}_{2}}O\]to \[{{H}_{3}}{{O}^{+}}\]
done
clear
C)
\[C{{H}_{4}}\]to\[{{C}_{2}}{{H}_{6}}~\]
done
clear
D)
\[B{{F}_{3}}\]to \[BF_{4}^{-}\]
done
clear
View Answer play_arrow
question_answer 92) In presence of \[HCl\], \[{{H}_{2}}S\] results the precipitation of group-2 elements but not group-4 elements during qualitative analysis. It is due to
A)
higher concentration of \[{{H}^{+}}\]
done
clear
B)
lower concentration of \[{{H}^{+}}\]
done
clear
C)
higher concentration of \[{{S}^{2-}}\]
done
clear
D)
lower concentration of \[{{S}^{2-}}\].
done
clear
View Answer play_arrow
question_answer 93) The two electrons have the following sets of quantum numbers: \[P=3,2,-2,+\frac{1}{2},\,\,Q=3,\,0,\,0,+\frac{1}{2}\] Which of the following statements is true?
A)
P has greater energy than Q.
done
clear
B)
P and Q represent same electron.
done
clear
C)
P and Q have same energy.
done
clear
D)
P has lesser energy than Q.
done
clear
View Answer play_arrow
question_answer 94) Orion has monomeric unit
A)
glycol
done
clear
B)
isoprene
done
clear
C)
acrolein
done
clear
D)
vinyl cyanide.
done
clear
View Answer play_arrow
question_answer 95) Adenosine is an example of
A)
purine base
done
clear
B)
nucleoside
done
clear
C)
nucleotide
done
clear
D)
pyrimidine base.
done
clear
View Answer play_arrow
question_answer 96) While charging the lead storage battery
A)
\[PbS{{O}_{4}}\]on cathode is reduced to \[Pb\]
done
clear
B)
\[PbS{{O}_{4}}\] on anode is oxidized to \[Pb{{O}_{2}}\]
done
clear
C)
\[PbS{{O}_{4}}\] on anode is reduced to \[Pb\]
done
clear
D)
\[PbS{{O}_{4}}\] on cathode is oxidized to \[Pb\].
done
clear
View Answer play_arrow
question_answer 97) The unit cell with crystallographic dimensions, \[a\ne b\ne c,\] \[\alpha =\gamma ={{90}^{o}}\] and \[\beta \ne {{90}^{o}}\] is
A)
monoclinic
done
clear
B)
tetragonal
done
clear
C)
triclinic
done
clear
D)
orthorhombic.
done
clear
View Answer play_arrow
question_answer 98) Sodium metal crystallizes in bcc lattice with edge length of\[4.29\overset{\text{o}}{\mathop{\text{A}}}\,\]. The radius of sodium atom is
A)
\[1.601\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1.857\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[2.857\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[2.145\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 99) On heating with concentrated \[NaOH\] solution in an inert atmosphere of \[C{{O}_{2}}\], white phosphorus gives a gas. Which of the following statements is incorrect about the gas?
A)
It is more basic than \[N{{H}_{3}}\]
done
clear
B)
Its solution in water decomposes in the presence of light.
done
clear
C)
It is less basic than \[N{{H}_{3}}\].
done
clear
D)
It is highly poisonous and has smell like rotten fish.
done
clear
View Answer play_arrow
question_answer 100) In the given set of reactions: 2-Bromopropane \[\xrightarrow[heat]{alc.\,\,AgCN}X\xrightarrow{LiAl{{H}_{4}}}Y\] The IUPAC name of product Y is
A)
N-isopropylmethanamine
done
clear
B)
N-methylpropan-2-amine
done
clear
C)
N-methylpropanamine
done
clear
D)
butan-2-amine.
done
clear
View Answer play_arrow
question_answer 101) \[{{H}_{2}}{{O}_{2}}\] cannot oxidise
A)
\[N{{a}_{2}}S{{O}_{3}}\]
done
clear
B)
\[KI\]
done
clear
C)
\[PbS\]
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 102) Which of the following will be able to show geometrical isomerism?
A)
\[M{{A}_{2}}{{B}_{2}}\]-Tetrahedral
done
clear
B)
\[MABCD\]-Tetrahedral
done
clear
C)
\[M{{A}_{3}}B\]-Square planar
done
clear
D)
\[MABCD\]-Square planar
done
clear
View Answer play_arrow
question_answer 103) Copper is extracted from copper pyrites by heating in a Bessemer converter. The method is based on the principle that
A)
iron has less affinity for oxygen than sulphur at high temperature
done
clear
B)
sulphur has less affinity for oxygen at high temperature
done
clear
C)
copper has more affinity for oxygen than sulphur at high temperature
done
clear
D)
copper has less affinity for oxygen than sulphur at high temperature.
done
clear
View Answer play_arrow
question_answer 104) The electrolyte having maximum flocculation value for \[AgI/A{{g}^{+}}\] sol is
A)
\[N{{a}_{2}}S\]
done
clear
B)
\[N{{a}_{3}}P{{O}_{4}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 105) In a first order reaction, the concentration of the reactant is reduced to 12.5% in one hour. When was it half completed?
A)
\[20min\]
done
clear
B)
\[15min\]
done
clear
C)
\[3hr\]
done
clear
D)
\[30min\]
done
clear
View Answer play_arrow
question_answer 106) 0.06% (w/v) aqueous solution of urea is isotonic with
A)
0.6% glucose solution
done
clear
B)
0.1 M glucose solution
done
clear
C)
0.06% glucose solution
done
clear
D)
0.01 M glucose solution.
done
clear
View Answer play_arrow
question_answer 107) In \[{{H}_{2}}-{{O}_{2}}\] fuel cell the reaction occurring at cathode is
A)
\[{{O}_{2(g)}}+2{{H}_{2}}{{O}_{(l)}}+4{{e}^{-}}\xrightarrow{{}}4OH_{(aq)}^{-}\]
done
clear
B)
\[H_{(aq)}^{+}+OH_{(aq)}^{-}\xrightarrow{{}}{{H}_{2}}{{O}_{(l)}}\]
done
clear
C)
\[2{{H}_{2(g)}}+{{O}_{2(g)}}\xrightarrow{{}}2{{H}_{2}}{{O}_{(l)}}\]
done
clear
D)
\[{{H}^{+}}+{{e}^{-}}\xrightarrow{{}}\frac{1}{2}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 108) The distinguishing test between methanoic acid and ethanoic acid is
A)
Tollenst -
done
clear
B)
sodium bicarbonate test
done
clear
C)
litmus test
done
clear
D)
esterification test.
done
clear
View Answer play_arrow
question_answer 109) The hydrolysis of optically active 2-bromobutane with aqueous \[NaOH\] results in the formation of
A)
\[(-)-butan-2-ol\]
done
clear
B)
\[(\pm )-butan-2-ol\]
done
clear
C)
\[(+)-butan-2-ol\]
done
clear
D)
\[(\pm )-butan-1-ol\]
done
clear
View Answer play_arrow
question_answer 110) \[MS{{O}_{4}}\xrightarrow{N{{H}_{4}}OH}\underset{white}{\mathop{X\downarrow }}\,\xrightarrow[Excess]{N{{H}_{4}}OH}Y\xrightarrow{{{H}_{2}}S}Z\downarrow \]Here M and Z are
A)
\[Zn,ZnS\]
done
clear
B)
\[Al,A{{l}_{2}}{{S}_{3}}\]
done
clear
C)
\[Cu,ZnS\]
done
clear
D)
\[Fe,FeS\]
done
clear
View Answer play_arrow
question_answer 111) The electronic configuration of \[G{{d}^{2+}}\] is (at. no. of Gd is 64)
A)
\[[Xe]4{{f}^{7}}\]
done
clear
B)
\[[Xe]\,4{{f}^{7}}\,5{{d}^{1}}\]
done
clear
C)
\[[Xe]\,4{{f}^{8}}\]
done
clear
D)
\[[Xe]\,4{{f}^{7}}\,\,5{{d}^{1}}\,\,6{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 112) Number of possible alkynes with formula \[{{C}_{5}}{{H}_{8}}\]is
A)
\[3\]
done
clear
B)
\[5\]
done
clear
C)
\[2\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 113) Glycogen is
A)
a structural polysaccharide
done
clear
B)
structurally similar to amytopectin but extensively branched
done
clear
C)
a polymer of \[\beta \]-D-glucose units
done
clear
D)
structurally very much similar to amylopectin.
done
clear
View Answer play_arrow
question_answer 114) How many ions per molecule are produced in the solution when Mohr salt is dissolved in excess of water?
A)
\[5\]
done
clear
B)
\[10\]
done
clear
C)
\[4\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 115) Which of the following curves is in accordance with Freundlich adsorption isotherm?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 116)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 117) On heating potassium permanganate, one of the following compound is not obtained
A)
\[MnO\]
done
clear
B)
\[{{K}_{2}}Mn{{O}_{4}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[Mn{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 118) The salt which responds to dilute and concentrated \[{{H}_{2}}S{{O}_{4}}\] is
A)
\[Ba{{(N{{O}_{3}})}_{2}}\]
done
clear
B)
\[N{{a}_{3}}P{{O}_{4}}\]
done
clear
C)
\[Ca{{F}_{2}}\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 119) Half-life period of a first order reaction is 10 min. Starting with initial concentration 12 M, the rate after 20 min is:
A)
\[0.693\times 3M\text{ }mi{{n}^{-1}}\]
done
clear
B)
\[0.0693\times 4M\text{ }mi{{n}^{-1}}\]
done
clear
C)
\[0.0693M\text{ }mi{{n}^{-1}}\]
done
clear
D)
\[0.0693\times 3M\text{ }mi{{n}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 120) Which of the following aqueous solutions has the highest freezing point?
A)
\[0.01M\text{ }NaCl\]
done
clear
B)
\[0.01M\text{ }N{{a}_{2}}S{{O}_{4}}\]
done
clear
C)
\[0.1M\]Sucrose
done
clear
D)
\[0.1M\text{ }NaCl\]
done
clear
View Answer play_arrow
question_answer 121) Which of the following sentences is correct?
A)
In prokaryotes there are no membrane bound cell organelles.
done
clear
B)
Cells of all living organisms have a nucleus.
done
clear
C)
Cells are formed dc novo from abiotic materials.
done
clear
D)
Both animal and plant cells have a well-defined cell wall.
done
clear
View Answer play_arrow
question_answer 122) The element responsible for the ring structure of chlorophyll and maintenance of ribosome structure is
A)
\[C{{a}^{++}}\]
done
clear
B)
\[M{{g}^{+}}\]
done
clear
C)
\[S\]
done
clear
D)
\[{{K}^{+}}\]
done
clear
View Answer play_arrow
question_answer 123) One hormone hastens maturity period in juvenile conifers, a second hormone controls xylem differentiation, while the third increases the tolerance of plants to various stresses. They are respectively
A)
Gibberellin, Auxin, Cytokinin
done
clear
B)
Auxin, Gibberellin, Cytokinin
done
clear
C)
Gibberellin, Auxin, ABA
done
clear
D)
Auxin, Gibberellin, ABA.
done
clear
View Answer play_arrow
question_answer 124) Which of the following is not an ex-situ conservation? .
A)
Cryopreservation
done
clear
B)
Seed bank
done
clear
C)
Biosphere reserves
done
clear
D)
Botanical garden
done
clear
View Answer play_arrow
question_answer 125) If 30J of energy is trapped at producer level, then how much energy will be available to peacock as food in the following chain? \[Plant\to Mice\to Snake\text{ }\to Peacock\]
A)
\[0.3J\]
done
clear
B)
\[0.03J\]
done
clear
C)
\[0.0003J\]
done
clear
D)
\[0.003J\]
done
clear
View Answer play_arrow
question_answer 126) With respect to phenylketonuria identify which statement is not correct.
A)
It is a case of aneuploidy.
done
clear
B)
It is an example of pleiotropy.
done
clear
C)
Caused due to autosomal recessive trait.
done
clear
D)
It is an error in metabolism.
done
clear
View Answer play_arrow
question_answer 127) In a human foetus the limbs and digits develop after
A)
12 weeks
done
clear
B)
first trimester
done
clear
C)
5th month
done
clear
D)
8 weeks.
done
clear
View Answer play_arrow
question_answer 128) The 2000 year old seed excavated from King Herods palace at dead sea belong to
A)
Dendrocalamus strictus
done
clear
B)
Lupine articus
done
clear
C)
Phoenix dactylifera
done
clear
D)
Strobilanthus kunthiana.
done
clear
View Answer play_arrow
question_answer 129)
Label the correct part of the Myosin monomer.
A)
[A] Actin binding site [B] Head [C] ATP binding site [D] Cross arm
done
clear
B)
[A] Cross arm [B] Actin binding site [C] Head [D] ATP binding site
done
clear
C)
[A] ATP binding site [B] Actin binding site [C] Head [D] Cross arm
done
clear
D)
[A] Head [B] Cross arm [C] Actin binding site [D] ATP binding site
done
clear
View Answer play_arrow
question_answer 130) Greenhouse crops such as tomatoes and bell pepper produce higher yields. This is due to
A)
\[C{{O}_{2}}\] enriched atmosphere leads to higher yields
done
clear
B)
\[C{{O}_{2}}\] is a limiting factor to photosynthesis
done
clear
C)
diffused light in green house
done
clear
D)
tomatoes and bell pepper are not \[{{C}_{3}}\] plants.
done
clear
View Answer play_arrow
question_answer 131) The organisms which completely lack a cell wall and can live without oxygen are
A)
mycoplasmas
done
clear
B)
archaebacteria
done
clear
C)
methanogens
done
clear
D)
thermoacidophiles.
done
clear
View Answer play_arrow
question_answer 132) RNA polymerase-I transcribes eukaryotic ribosome which does not consist of
A)
\[5.8S\text{ }rRNA\]
done
clear
B)
\[28S\text{ }rRNA\]
done
clear
C)
\[18S\text{ }rRNA\]
done
clear
D)
\[5S\text{ }rRNA\].
done
clear
View Answer play_arrow
question_answer 133)
Match the following. [A] VNTR p. Largest gene [B] Introns and Exons q. DNA fingerprinting [C] Dystrophin r. Bulk DNA [D] Satellite DNA s. Splicing
A)
\[\text{(A)}-r,(B)-\text{ }s,(C)-\text{ }p,(D)-\text{ }q\]
done
clear
B)
\[\text{(A)}-q,(B)-\text{ }s,(C)-\text{ }p,(D)-r\]
done
clear
C)
\[\text{(A)}-q,(B)-p,(C)-\text{s},(D)-r\]
done
clear
D)
\[\text{(A)}-s,(B)-p,(C)-q,(D)-r\]
done
clear
View Answer play_arrow
question_answer 134) Smack and Crack are produced from
A)
Cannabis saliva and Papaver somniferum
done
clear
B)
Cannabis saliva and Atropa belladonna
done
clear
C)
Erythroxylon coca and Atropa belladonna
done
clear
D)
Papaver somniferum and Erythroxylon coca.
done
clear
View Answer play_arrow
question_answer 135) Double lines in pedigree analysis show
A)
unaffected offspring
done
clear
B)
sex unspecified
done
clear
C)
normal mating
done
clear
D)
consanguineous marriage.
done
clear
View Answer play_arrow
question_answer 136) Progestasert is an IUD which makes the uterus unsuitable and cervix hostile to the sperms as they are
A)
hormone releasing lUDs
done
clear
B)
copper releasing lUDs
done
clear
C)
ideal contraceptive
done
clear
D)
non-medicated lUDs.
done
clear
View Answer play_arrow
question_answer 137) The chromosome number in meiocyte is 34. Therganism could be
A)
Ophioglossum
done
clear
B)
dog
done
clear
C)
onion
done
clear
D)
apple.
done
clear
View Answer play_arrow
question_answer 138) A fall in glomerular filtration rate activates
A)
adrenal medulla to release adrenaline
done
clear
B)
juxtaglomerular cells to release renin
done
clear
C)
posterior pituitary to release vasopressin
done
clear
D)
adrenal cortex to release aldosterone.
done
clear
View Answer play_arrow
question_answer 139) In a \[3.2Kbp\]long piece of DNA, 820 adenine bases were found. What would be the number of cytosine bases?
A)
\[780\]
done
clear
B)
\[1560\]
done
clear
C)
\[740\]
done
clear
D)
\[1480\]
done
clear
View Answer play_arrow
question_answer 140) Assisted Reproductive Technology does not include
A)
Zygote Extra Fallopian Transfer
done
clear
B)
In vitro fertilisation and embryo transfer
done
clear
C)
artificial insemination
done
clear
D)
Gamete Intra Fallopian Transfer.
done
clear
View Answer play_arrow
question_answer 141) During menstrual cycle, the cyclical changes take place in
A)
perimetrium
done
clear
B)
endometrium
done
clear
C)
corpus luteum
done
clear
D)
myometrium.
done
clear
View Answer play_arrow
question_answer 142) BOD refers to
A)
the oxygen required for bacteria to grow in 1 Litre of effluent.
done
clear
B)
the amount of oxygen consumed if all the organic matter in 1000 mL of water were oxidised by bacteria
done
clear
C)
the amount of oxygen released if all the organic matter in 1000 mL of water were oxidised by bacteria
done
clear
D)
the amount of oxygen released when all the organic matter was consumed by bacteria in 1 litre of water
done
clear
View Answer play_arrow
question_answer 143) Sonalika and Kalyan Sona are high yielding varieties of
A)
sugarcane
done
clear
B)
rice
done
clear
C)
wheat
done
clear
D)
maize.
done
clear
View Answer play_arrow
question_answer 144) According to Robert Constanza, 50% of the total cost for ecosystem services goes to
A)
nutrient cycling
done
clear
B)
recreation
done
clear
C)
soil formation
done
clear
D)
climate regulation.
done
clear
View Answer play_arrow
question_answer 145) Choose the correct statement.
A)
Oxygen is vital in respiration for removal of hydrogen.
done
clear
B)
Pyruvate is formed in the mitochondrial matrix.
done
clear
C)
There is complete breakdown of glucose in fermentation.
done
clear
D)
During the conversion of succinyl CoA to succinic acid a molecule of ATP is synthesised.
done
clear
View Answer play_arrow
question_answer 146)
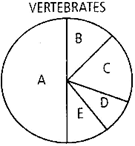
Given below is the representation of the extent of global diversity of vertebrates. What groups does the portions represent?
A)
A B C D E Birds Reptiles Fishes Mammals Amphibians
done
clear
B)
A B C D E Mammals Birds Fishes Amphibians Reptiles
done
clear
C)
A B C D E Fishes Amphibians Mammals Birds Reptiles
done
clear
D)
A B C D E Fishes Mammals Birds Reptiles Amphibians
done
clear
View Answer play_arrow
question_answer 147) Select the mismatch pair from the following.
A)
Oxytocin - Contraction of uterine muscles
done
clear
B)
Insulin - Gluconeogenesis
done
clear
C)
Prolactin - Milk production in mammary glands
done
clear
D)
Glucagon - Glycogenolysis
done
clear
View Answer play_arrow
question_answer 148) Which of the following would most likely help to slow down the greenhouse effect?
A)
Converting tropical forests into grazing land for cattle.
done
clear
B)
Ensuring that all excess paper packaging is burned to ashes.
done
clear
C)
Redesigning landfill dumps to allow methane to be collected.
done
clear
D)
Promoting the use of-private rather than public transport.
done
clear
View Answer play_arrow
question_answer 149) If an inheritable mutation is observed in a population at high frequency, it is referred to as 0risinal Question is incorrectly framed
A)
sequence annotation
done
clear
B)
DNA polymorphism
done
clear
C)
linkage
done
clear
D)
expressed sequence tag.
done
clear
View Answer play_arrow
question_answer 150) Find the wrongly matched pair.
A)
Lungs of the Amazon rainforest planet
done
clear
B)
Endemism Species confined to one region and also found in other regions
done
clear
C)
Hotspots Regions with species richness
done
clear
D)
Alien species Glorias gariepinus
done
clear
View Answer play_arrow
question_answer 151) The function of a selectable marker is
A)
eliminating transformants and permitting non-transformants
done
clear
B)
identify ori site
done
clear
C)
elimination of non-transformants and permitting transformants
done
clear
D)
to destroy recognition sites.
done
clear
View Answer play_arrow
question_answer 152) The T-wave in an ECG represents
A)
depolarisation of ventricles
done
clear
B)
electrical excitation of atria
done
clear
C)
beginning of systole
done
clear
D)
return of the ventricles from excited state.
done
clear
View Answer play_arrow
question_answer 153) In prokaryotes the glycocalyx when it is thick is called
A)
capsule
done
clear
B)
slime layer
done
clear
C)
cell wall
done
clear
D)
mesosome.
done
clear
View Answer play_arrow
question_answer 154) Which of the following is not correct in mass flow hypothesis?
A)
As hydrostatic pressure in the phloem sieve tube increases pressure flow stops and sap is accumulated in phloem.
done
clear
B)
The sugar is moved bidirectionally.
done
clear
C)
The sugar which is transported is sucrose.
done
clear
D)
Loading of the phloem sets up a water potential gradient that facilitates the mass movement in the phloem.
done
clear
View Answer play_arrow
question_answer 155)
Identify this structure.
A)
Adenylic acid
done
clear
B)
Uracil
done
clear
C)
Cholesterol
done
clear
D)
Adenosine
done
clear
View Answer play_arrow
question_answer 156) In 125 amino acid sequence if the codon for 25th amino acid is mutated to UAA, then
A)
a polypeptide of 24 amino acids is formed
done
clear
B)
a polypeptide of 124 amino acids is formed
done
clear
C)
no polypeptides are formed
done
clear
D)
a polypeptide of 25 amino acids is formed.
done
clear
View Answer play_arrow
question_answer 157) Three copies of chromosome - 21 in a child with Downs syndrome have been analysed using molecular biology technology to detect any possible DNA polymorphism with reference to different alleles located on chromosome - 21. Results showed that out of 3 copies 2 of the chromosomes of the child contain the same alleles as one of the mother s alleles. Based on this when did the non-disjunction event most likely occur?
A)
Paternal meiosis-I
done
clear
B)
Maternal meiosis-I
done
clear
C)
Paternal meiosis-II
done
clear
D)
Maternal meiosis-II
done
clear
View Answer play_arrow
question_answer 158) Which of the following is not correct with respect to malaria?
A)
RBCs rupture and release haemozoin which causes chills.
done
clear
B)
Sporozoites multiply in blood.
done
clear
C)
Female Anopheles mosquito is the vector.
done
clear
D)
Malignant malaria is caused by Plasmodium falciparum.
done
clear
View Answer play_arrow
question_answer 159) Ernst Chain and Howard Floreys contribution was
A)
establishing the potential of penicillin as an effective antibiotic
done
clear
B)
discovery of streptokinase
done
clear
C)
production of genetically engineered insulin
done
clear
D)
discovery of DNA sequence.
done
clear
View Answer play_arrow
question_answer 160)
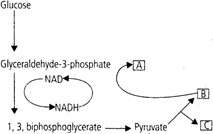
Choose the correct combination of labelling the molecules involved in the pathway of anaerobic respiration in Yeast.
A)
A - Acetaldehyde, B -\[C{{O}_{2}}\] C - Ethanol
done
clear
B)
A - Ethanol, B - \[C{{O}_{2}}\], C - Acetaldehyde
done
clear
C)
A - Ethanol, B - Acetaldehyde, C - \[C{{O}_{2}}\]
done
clear
D)
A - \[C{{O}_{2}}\], B - Ethanol, C - Acetaldehyde
done
clear
View Answer play_arrow
question_answer 161) The breakdown of detritus into small particles by detritivores is called
A)
leaching
done
clear
B)
humification
done
clear
C)
fragmentation
done
clear
D)
catabolism.
done
clear
View Answer play_arrow
question_answer 162) The formation of two species from one ancestral species is known as
A)
convergent evolution
done
clear
B)
phyletic evolution
done
clear
C)
allopatry
done
clear
D)
divergent evolution.
done
clear
View Answer play_arrow
question_answer 163) A scrubber in the exhaust of a chemical industrial plant removes
A)
gases like ozone or methane
done
clear
B)
gases like sulphur dioxide
done
clear
C)
gases like nitrous oxide
done
clear
D)
particulate matter of the size 5 micrometers or above.
done
clear
View Answer play_arrow
question_answer 164) With respect to DNA fragmentation Statement A: Gel electrophoresis and elution are two important processes. Statement B: After staining with ethidium bromide it has to be exposed to U.V. light.
A)
Only A is correct.
done
clear
B)
Both A and B are correct statements.
done
clear
C)
Only B is correct.
done
clear
D)
Only A is correct and B is not correct.
done
clear
View Answer play_arrow
question_answer 165) The pioneer species in xerarch and hydrarch succession are respectively
A)
lichens and phytoplanktons
done
clear
B)
lichens and sedges
done
clear
C)
phytoplanktons and lichens
done
clear
D)
lichens and rooted hydrophytes.
done
clear
View Answer play_arrow
question_answer 166) Hibernating animals have tissues containing mitochondria with a membrane protein that accelerates electron transport while blocking the synthesis of ATR What is the consequence of this?
A)
Hibernating animals can synthesise fat instead of wasting energy of respiration,
done
clear
B)
Energy is saved because glycolysis and the citric acid cycle shuts down.
done
clear
C)
Pyruvate is converted to lactic acid by anaerobic fermentation.
done
clear
D)
The energy of respiration is converted into heat.
done
clear
View Answer play_arrow
question_answer 167) Which of the following conditions correctly describes the manner of determining the sex in the given example?
A)
Homozygous sex chromosome XX produces male in Drosophila.
done
clear
B)
XO type of sex determines male sex in grasshopper.
done
clear
C)
Homozygous sex chromosome ZZ determines female sex in birds.
done
clear
D)
XO condition in humans as found in Klinefelters syndrome determines female sex.
done
clear
View Answer play_arrow
question_answer 168) Natural killer lymphocytes are an example for
A)
physical barrier
done
clear
B)
cytokine barrier
done
clear
C)
cellular barrier
done
clear
D)
physiological barrier.
done
clear
View Answer play_arrow
question_answer 169) The codon AUG has dual function. It is an initiation codon and also codes for
A)
phenylalanine
done
clear
B)
formaldehyde
done
clear
C)
serine
done
clear
D)
methionine.
done
clear
View Answer play_arrow
question_answer 170) Identify the wrong statement.
A)
Alleles b and c also produce sugar.
done
clear
B)
Alleles \[{{I}^{A}}\] and \[{{I}^{B}}\] produce sugars.
done
clear
C)
When \[{{I}^{B}}\] and b or i are present only \[{{I}^{B}}\] is expressed.
done
clear
D)
Both \[{{I}^{A}}\] and \[{{I}^{B}}\] are present together and they express because of co-dominance.
done
clear
View Answer play_arrow
question_answer 171) Continued self-pollination results in
A)
formation of unisexual flowers
done
clear
B)
inbreeding depression
done
clear
C)
gametes loose vigour
done
clear
D)
self incompatibility.
done
clear
View Answer play_arrow
question_answer 172) Which vector can clone a small fragment of DNA?
A)
Plasmid
done
clear
B)
Bacterial artificial chromosome
done
clear
C)
Cosmid
done
clear
D)
Yeast artificial chromosome
done
clear
View Answer play_arrow
question_answer 173) Seeds without fertilisation are obtained from
A)
polyembryony
done
clear
B)
parthenocarpy
done
clear
C)
dormancy
done
clear
D)
apomixis.
done
clear
View Answer play_arrow
question_answer 174) With respect to Eichhornia: Statement X: It drains offoxvgen from water and is seen growing in standing water. Statement Y: It is an indigenous species of our country.
A)
Only statement X is correct and Y is wrong.
done
clear
B)
Both statements X and Y are correct.
done
clear
C)
Only statement Y is correct and X is wrong.
done
clear
D)
Both statements X and Y are wrong.
done
clear
View Answer play_arrow
question_answer 175)
Identify the Phylum X.
A)
Hemichordata
done
clear
B)
Aschelminthes
done
clear
C)
Platyhelminthes
done
clear
D)
Ctenophora
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
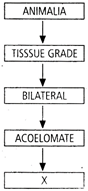
question_answer 176) During sewage treatment biogas produced includes
A)
hydrogen sulphide, nitrogen, methane
done
clear
B)
methane, oxygen, hydrogen sulphide
done
clear
C)
methane, hydrogen sulphide, carbon dioxide
done
clear
D)
hydrogen sulphide, methane, sulphur oxide.
done
clear
View Answer play_arrow
question_answer 177) The ancestors of modern day frogs and salamanders are
A)
Icthyophis
done
clear
B)
jawless fish
done
clear
C)
Amphioxus
done
clear
D)
Coelacanth.
done
clear
View Answer play_arrow
question_answer 178) Hisardale is obtained by crossing
A)
horse with donkey
done
clear
B)
Merino ewes with Bikaneri rams
done
clear
C)
superior bull with superior cow
done
clear
D)
Bikaneri ewes with Merino rams.
done
clear
View Answer play_arrow
question_answer 179) The nitrogen base found only in DNA is also called
A)
uracil
done
clear
B)
5-methyl uracil
done
clear
C)
guanine
done
clear
D)
\[N{{H}_{4}}Cl\]
done
clear
View Answer play_arrow
question_answer 180) The hormone which acts on Sertoli cells and stimulates the process of spermiogenesis is
A)
GnRH
done
clear
B)
androgen
done
clear
C)
FSH
done
clear
D)
LH.
done
clear
View Answer play_arrow
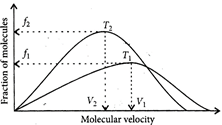 Which of the following is correct about this plot?
Which of the following is correct about this plot?
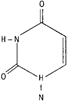
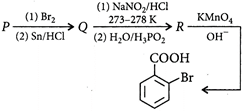 The starting compound P is
The starting compound P is