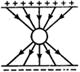
question_answer 1) The difference between the apparent frequency of a source of sound as perceived by the observer during its approach and recession is 2% of the frequency of the source. If the speed of sound in air is 300 ms-1, the velocity of the source is:
A)
\[1.5\text{ }m{{s}^{-1}}\]
done
clear
B)
\[12\text{ }m{{s}^{-1}}\]
done
clear
C)
\[6\text{ }m{{s}^{-1}}\]
done
clear
D)
\[3\text{ }m{{s}^{-1}}\]
done
clear
View Answer play_arrow
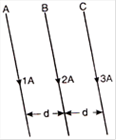
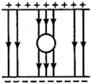
question_answer 2)
Three long straight wires A, B and C are carrying currents as shown in figure. Then the resultant force on B is directed:
A)
perpendicular to the plane of paper and outward
done
clear
B)
perpendicular to the plane of paper and inward
done
clear
C)
towards A
done
clear
D)
towards C
done
clear
View Answer play_arrow
question_answer 3) Curie - Weiss law is obeyed by iron:
A)
at Curie temperature only
done
clear
B)
at all temperatures
done
clear
C)
below Curie temperature
done
clear
D)
above Curie temperature
done
clear
View Answer play_arrow
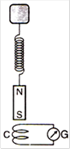
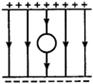
question_answer 4)
A magnet N-S is suspended from a spring and when it oscillates, the magnet moves in and out of the coil C. The coil is connected to a galvanometer G. Then, as the magnet oscillates:
A)
G shows no deflection
done
clear
B)
G shows deflection to the left and right but the amplitude steadily decreases
done
clear
C)
G Shows deflection to the left and right with constant amplitude
done
clear
D)
G shows deflection on one side.
done
clear
View Answer play_arrow
question_answer 5) The dimensional formula for inductance is:
A)
\[\left[ M{{L}^{2}}{{T}^{-2}}{{A}^{-2}} \right]\]
done
clear
B)
\[\left[ M{{L}^{2}}T{{A}^{-2}} \right]\]
done
clear
C)
\[\left[ M{{L}^{2}}{{T}^{-1}}{{A}^{-2}} \right]\]
done
clear
D)
\[\left[ M{{L}^{2}}{{T}^{-2}}{{A}^{-1}} \right]\]
done
clear
View Answer play_arrow
question_answer 6) The maximum current that can be measured by a galvanometer of resistance \[40\,\,\Omega \], is 10 mA. It is converted into a voltmeter that can read upto 50 V. The resistance to be connected in series with the galvanometer (in ohms) is:
A)
2010
done
clear
B)
4050
done
clear
C)
5040
done
clear
D)
4960
done
clear
View Answer play_arrow
question_answer 7) The spectrum obtained from the chromosphere of the sun at the time of total solar eclipse is:
A)
line emission spectrum
done
clear
B)
band emission spectrum
done
clear
C)
continuous emission spectrum
done
clear
D)
line absorption spectrum
done
clear
View Answer play_arrow
question_answer 8) Heavy water is:
A)
compound of deuterium and oxygen
done
clear
B)
water at \[{{4}^{o}}C\]
done
clear
C)
water, in which soap does not lather
done
clear
D)
compound of heavy oxygen and heavy hydrogen
done
clear
View Answer play_arrow
question_answer 9) The nuclear reactor at Kaiga is a:
A)
research reactor
done
clear
B)
fusion reactor
done
clear
C)
breeder reactor
done
clear
D)
power reactor
done
clear
View Answer play_arrow
question_answer 10) When a body moves in a circular path, no work is done by the force since:
A)
force and displacement are perpendicular to each other
done
clear
B)
the force is always away from the centre
done
clear
C)
there is no displacement
done
clear
D)
there is no net force
done
clear
View Answer play_arrow
question_answer 11) A bullet moving with a speed of \[100\text{ }m{{s}^{-1}}\] can just penetrate two planks of equal thickness. Then, the number of such planks penetrated by the same bullet when the speed is doubled will be:
A)
6
done
clear
B)
10
done
clear
C)
4
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 12) Two bodies of masses 1 kg and 2 kg have equal momentum. Then, the ratio of their kinetic energies is:
A)
\[2:1\]
done
clear
B)
\[3:1\]
done
clear
C)
\[1:3\]
done
clear
D)
\[1:1\]
done
clear
View Answer play_arrow
question_answer 13) Absorption coefficient of an open window is:
A)
1
done
clear
B)
0.25
done
clear
C)
zero
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 14) The loudness and pitch of a sound note depend on:
A)
intensity and velocity
done
clear
B)
frequency and velocity
done
clear
C)
intensity and frequency
done
clear
D)
frequency and number of harmonics
done
clear
View Answer play_arrow
question_answer 15) In Meldes experiment in the transverse mode, the frequency of the tuning fork and the frequency of the waves in the string are in the ratio:
A)
\[2:1\]
done
clear
B)
\[4:1\]
done
clear
C)
\[1:1\]
done
clear
D)
\[1:2\]
done
clear
View Answer play_arrow
question_answer 16) An electron is accelerated through a potential difference of 45.5 volt. The velocity acquired by it is (in \[m{{s}^{-1}}\]):
A)
\[{{10}^{6}}\]
done
clear
B)
zero
done
clear
C)
\[4\times {{10}^{6}}\]
done
clear
D)
\[4\times {{10}^{4}}\]
done
clear
View Answer play_arrow
question_answer 17) An unknown resistance R1 is connected in series with a resistance of \[10\,\Omega \]. This combination is connected to one gap a metre bridge while a resistance \[{{R}_{2}}\] is connected in the other gap. The balance point is at 50 cm. Now, when the \[10\,\Omega \] resistance is removed the balance point shifts to 40 cm. The value of \[{{R}_{1}}\] is (in ohms):
A)
20
done
clear
B)
10
done
clear
C)
60
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 18)
In the circuit shown, the internal resistance 3f the cell is negligible. The steady state current in the \[2\,\Omega \] resistor is:
A)
0.6 A
done
clear
B)
1.2 A
done
clear
C)
0.9 A
done
clear
D)
1.5 A
done
clear
View Answer play_arrow
question_answer 19) A rectangular coil of 300 turns has an average area of \[25\text{ }cm\times 10\text{ }cm\]. The coil rotates with a speed of 50 cps in uniform magnetic field of strength \[4\times {{10}^{-2}}T\]about an axis perpendicular to the field. The peak value of the induced emf is (in volt)
A)
\[300\,\pi \]
done
clear
B)
\[3000\,\pi \]
done
clear
C)
\[3\,\pi \]
done
clear
D)
\[30\,\pi \]
done
clear
View Answer play_arrow
question_answer 20) In an LCR circuit the potential difference between the terminals of the inductance is 60 V, between the terminals of the capacitor is 30 V and that between the terminals of resistance is 40 V. The supply voltage will be equal to:
A)
130 V
done
clear
B)
10 V
done
clear
C)
50 V
done
clear
D)
70 V
done
clear
View Answer play_arrow
question_answer 21) A vertical circular coil of radius 0.1 m and having 10 turns carries a steady current. When the plane of the coil is normal to the magnetic meridian, a neutral point is observed at the centre of the coil. If\[{{B}_{H}}=0.314\times {{10}^{-4}}T\], the current in the coil is:
A)
0.5 A
done
clear
B)
0.25 A
done
clear
C)
2 A
done
clear
D)
1 A
done
clear
View Answer play_arrow
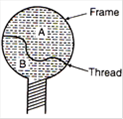
question_answer 22)
A thread is tied slightly loose to a wire frame as in figure and the frame is dipped into a soap solution and taken out. The frame is completely covered with the film. When the portion A is punctured with a pin, the thread:
A)
becomes concave towards A
done
clear
B)
becomes convex towards A
done
clear
C)
either (a) or (b) depending on the size of A with respect to B
done
clear
D)
remains in the initial position
done
clear
View Answer play_arrow
question_answer 23) Oxygen is 16 times heavier than hydrogen. Equal volumes of hydrogen and oxygen are mixed. The ratio of speed of sound in the mixture to that in hydrogen is:
A)
\[\sqrt{8}\]
done
clear
B)
\[\sqrt{2/17}\]
done
clear
C)
\[\sqrt{1/8}\]
done
clear
D)
\[\sqrt{32/17}\]
done
clear
View Answer play_arrow
question_answer 24) If two waves of the same frequency and amplitude respectively on superposition produce a resultant disturbance of the same amplitude, the waves differ in phase by:
A)
\[\pi \]
done
clear
B)
zero
done
clear
C)
\[\pi /3\]
done
clear
D)
\[2\,\pi /3\]
done
clear
View Answer play_arrow
question_answer 25) A man, standing between two cliffs, claps his hands and starts hearing a series of echoes at intervals of one second. If the speed of sound in air is \[340\,m{{s}^{-1}}\], the distance between the cliffs is:
A)
680 m
done
clear
B)
1700 m
done
clear
C)
340 m
done
clear
D)
1620 m
done
clear
View Answer play_arrow
question_answer 26) A beam of light of wavelength 600 nm from a source falls on a single slit 1 mm wide and the resulting diffraction pattern is observed on a screen 2 m away. The distance between the first dark fringes on either side of the central bright fringe is:
A)
2.4 cm
done
clear
B)
2.4 mm
done
clear
C)
1.2 mm
done
clear
D)
1.2 cm
done
clear
View Answer play_arrow
question_answer 27) Specific rotation of sugar solution is units. \[200\text{ }kg{{m}^{-3}}\] of impure sugar solution is taken in a polarimeter tube of length 0.25 m and an optical rotation of 0.4 rad is observed. The percentage of purity of sugar in the sample is:
A)
11%
done
clear
B)
20%
done
clear
C)
80%
done
clear
D)
89%
done
clear
View Answer play_arrow
question_answer 28) The emitter - base junction of a transistor is ......... biased while the collector - base junction is ......... biased.
A)
forward, forward
done
clear
B)
forward, reverse
done
clear
C)
reverse, forward
done
clear
D)
reverse, reverse
done
clear
View Answer play_arrow
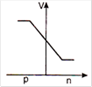
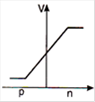
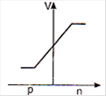
question_answer 29) In a forward biased p-n junction diode, the potential barrier in the depletion region is of the form:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 30) A cylinder of radius r and length \[l\] is placed in an uniform electric field E parallel to the axis of the cylinder. The total flux for the surface of the cylinder is given by:
A)
zero
done
clear
B)
\[2\pi {{r}^{2}}E\]
done
clear
C)
\[\pi {{r}^{2}}E\]
done
clear
D)
\[(\pi {{r}^{2}}+\pi {{l}^{2}})\,E\]
done
clear
View Answer play_arrow
question_answer 31) Two electric bulbs A and B are rated as 60 W and 100 W. They are connected in parallel to the same source. Then:
A)
B draws more current than A
done
clear
B)
currents drawn are in the ratio of their resistances
done
clear
C)
both draw the same current
done
clear
D)
A draws more current than B
done
clear
View Answer play_arrow
question_answer 32) A thin plano-convex lens acts like a concave mirror of focal length 0.2 m when silvered from its plane surface. The refractive index of the material of the lens is 1.5. The radius of curvature of the convex surface of the lens will be:
A)
0.1 m
done
clear
B)
0.75 m
done
clear
C)
0.4 m
done
clear
D)
0.2 m
done
clear
View Answer play_arrow
question_answer 33) The physical quantity having the same dimensions as Plancks constant h is:
A)
linear momentum
done
clear
B)
angular momentum
done
clear
C)
Boltzmann constant
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 34) A balloon is rising vertically up with a velocity of \[29\text{ }m{{s}^{-1}}\]. A stone is dropped from it and it reaches the ground in 10 seconds. The height of the balloon when the stone was dropped from it is \[(g=9.8\text{ }m{{s}^{-1}})\]:
A)
400 m
done
clear
B)
150 m
done
clear
C)
100 m
done
clear
D)
200 m
done
clear
View Answer play_arrow
question_answer 35) A wire has a resistance of \[6\,\Omega \]. It is cut into two parts and both half values are connected in parallel. The new resistance is:
A)
\[3\,\Omega \]
done
clear
B)
\[6\,\Omega \]
done
clear
C)
\[12\,\,\Omega \]
done
clear
D)
\[1.5\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 36) In a Youngs double slit experiment, the separation between the two slits is 0.9 mm and the fringes are observed one metre away. If it produces the second dark fringe at a distance of 1 mm from the central fringe, the wavelength of the monochromatic source of light used is:
A)
450 nm
done
clear
B)
400 nm
done
clear
C)
500 nm
done
clear
D)
600 nm
done
clear
View Answer play_arrow
question_answer 37) When light is incident on a diffraction grating, the zero order principal maximum will be:
A)
spectrum of the colours
done
clear
B)
white
done
clear
C)
one of the component colours
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 38) H-polaroid is prepared by:
A)
orienting herapathite crystal in the same direction in nitrocellulose
done
clear
B)
using thin tourmaline crystals
done
clear
C)
stretching polyvinyl alcohol and then heated with dehydrating agent
done
clear
D)
stretching polyvinyl alcohol and then impregnating with iodine
done
clear
View Answer play_arrow
question_answer 39) SI unit of permittivity is:
A)
\[{{C}^{2}}{{m}^{2}}{{N}^{2}}\]
done
clear
B)
\[{{C}^{2}}{{m}^{-2}}{{N}^{-1}}\]
done
clear
C)
\[{{C}^{2}}{{m}^{2}}{{N}^{-1}}\]
done
clear
D)
\[{{C}^{-1}}{{m}^{2}}{{N}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 40) A spherical drop of capacitance \[1\,\mu F\] is broken into eight drops of equal radius. Then, the capacitance of each small drop is:
A)
\[\frac{1}{2}\mu F\]
done
clear
B)
\[\frac{1}{4}\mu F\]
done
clear
C)
\[\frac{1}{8}\mu F\]
done
clear
D)
\[8\,\mu F\]
done
clear
View Answer play_arrow
question_answer 41) Two equal forces (P each) act at a point inclined to each other at an angle of \[{{120}^{o}}\]. The magnitude of their resultant is:
A)
\[P/2\]
done
clear
B)
\[P/4\]
done
clear
C)
P
done
clear
D)
\[2P\]
done
clear
View Answer play_arrow
question_answer 42) Threshold wavelength for photoelectric emission from a metal surface is \[5200\text{ }\overset{o}{\mathop{A}}\,\]. Photoelectrons will be emitted when this surface is illuminated with monochromatic radiation from:
A)
1 W IR lamp
done
clear
B)
50 W UV lamp
done
clear
C)
50 W IR lamp
done
clear
D)
10 W IR lamp
done
clear
View Answer play_arrow
question_answer 43) In Youngs double slit experiment if monochromatic light used is replaced by white light, then:
A)
no fringes are observed
done
clear
B)
only central fringe is white, all other fringes are colored
done
clear
C)
all bright fringes become white
done
clear
D)
all bright fringes have colours between violet and red
done
clear
View Answer play_arrow
question_answer 44) Which state of triply ionised Beryllium \[(B{{e}^{+++}})\] has the same orbital radius as that of the ground state of hydrogen?
A)
\[n=3\]
done
clear
B)
\[n=4\]
done
clear
C)
\[n=1\]
done
clear
D)
\[n=2\]
done
clear
View Answer play_arrow
question_answer 45) If M is the atomic mass and A is the mass number, packing fraction is given by:
A)
\[\frac{M}{M-A}\]
done
clear
B)
\[\frac{M-A}{A}\]
done
clear
C)
\[\frac{A}{M-A}\]
done
clear
D)
\[\frac{A-M}{A}\]
done
clear
View Answer play_arrow
question_answer 46) A count rate meter shows a count of 240 per minute from a given radioactive source. One hour later the meter shows a count rate of 30 per minute. The half-life of the source is:
A)
80 min
done
clear
B)
120 min
done
clear
C)
20 min
done
clear
D)
30 mm
done
clear
View Answer play_arrow
question_answer 47) Two conductors of the same material have their diameters in the ratio \[1:2\] and their lengths in the ratio \[2:1\]. If the temperature difference between their ends is the same, then the ratio of amounts of heat conducted per second through them will be:
A)
\[4:1\]
done
clear
B)
\[1:4\]
done
clear
C)
\[8:1\]
done
clear
D)
\[1:8\]
done
clear
View Answer play_arrow
question_answer 48) Blowing air with open mouth is an example of:
A)
isobaric process
done
clear
B)
isochoric process
done
clear
C)
isothermal process
done
clear
D)
adiabatic process.
done
clear
View Answer play_arrow
question_answer 49) Sound waves in air are always longitudinal because:
A)
of the inherent characteristics of sound waves in air
done
clear
B)
air does not have a modulus of rigidity
done
clear
C)
air is a mixture of several gases
done
clear
D)
density of air is very small
done
clear
View Answer play_arrow
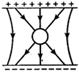
question_answer 50) An uncharged sphere of metal is placed inside a charged parallel plate capacitor. The lines of force will look like:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 51) A current flows in a conductor from east to west. The direction of the magnetic field at a point above the conductor is :
A)
towards east
done
clear
B)
towards west
done
clear
C)
towards north
done
clear
D)
towards south
done
clear
View Answer play_arrow
question_answer 52) A bar magnet is equivalent to :
A)
torroid carrying current
done
clear
B)
straight conductor carrying current
done
clear
C)
solenoid carrying current
done
clear
D)
circular coil carrying current.
done
clear
View Answer play_arrow
question_answer 53) Excitation energy of a hydrogen like ion in its first excitation state is 40.8 eV. Energy needed to remove the electron from the ion in ground state is :
A)
40.8 eV
done
clear
B)
27.2 eV
done
clear
C)
54.4 eV
done
clear
D)
13.6 eV
done
clear
View Answer play_arrow
question_answer 54) The refractive index of a particular material is 1.67 for blue light, 1.65 for yellow light and 1.63 for red light. The dispersive power of the material is :
A)
0.031
done
clear
B)
1.60
done
clear
C)
0.0615
done
clear
D)
0.024
done
clear
View Answer play_arrow
question_answer 55) An ideal gas heat engine operates in a Carnots cycle between \[{{227}^{o}}C\] and \[{{127}^{o}}C\]. It absorbs \[6\times {{10}^{4}}J\] at high temperature. The amount of heat converted into work is :
A)
\[1.6\times {{10}^{4}}J\]
done
clear
B)
\[1.2\times {{10}^{4}}J\]
done
clear
C)
\[4.8\times {{10}^{4}}J\]
done
clear
D)
\[3.5\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
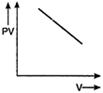
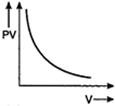
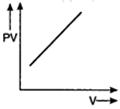
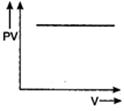
question_answer 56) Which one of the following graphs represents the behaviour of an ideal gas?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 57) Rainbow is formed due to :
A)
total internal reflection
done
clear
B)
scattering
done
clear
C)
refraction
done
clear
D)
dispersion and total internal reflection
done
clear
View Answer play_arrow
question_answer 58) A beam of parallel rays is brought to focus by a plano-convex lens. A thin concave lens of the same focal length is joined to the first lens. The effect of this is :
A)
the focus shifts to infinity
done
clear
B)
the focal point shifts towards the lens by a small distance
done
clear
C)
the focal point shifts away from die lens by a small distance
done
clear
D)
the focus remains undisturbed
done
clear
View Answer play_arrow
question_answer 59) When a body is earth connected, electrons from the earth flow into the body. This means the body is :
A)
charged negatively
done
clear
B)
an insulator
done
clear
C)
uncharged
done
clear
D)
charged positively
done
clear
View Answer play_arrow
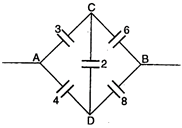
question_answer 60)
Effective capacitance between A and B in the figure shown is (all capacitances are in \[\mu F\]) :
A)
\[\frac{3}{14}\mu F\]
done
clear
B)
\[\frac{14}{3}\mu F\]
done
clear
C)
\[21\,\mu F\]
done
clear
D)
\[23\,\mu F\]
done
clear
View Answer play_arrow
question_answer 61) Which one of the following is not an amphoteric substance?
A)
\[HN{{O}_{3}}\]
done
clear
B)
\[HCO_{3}^{-}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 62) When \[50\text{ }c{{m}^{3}}\] of \[0.2\,N\,{{H}_{2}}S{{O}_{4}}\] is mixed with \[50\,\,c{{m}^{3}}\] of 1 N KOH, the heat liberated is:
A)
11.46 kJ
done
clear
B)
57.3 kJ
done
clear
C)
573 kJ
done
clear
D)
573 J
done
clear
View Answer play_arrow
question_answer 63) An artificial radioactive isotope gave 7 N after two successive P-particle emissions. The number of neutrons in the parent nucleus must be:
A)
9
done
clear
B)
14
done
clear
C)
5
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 64) Stainless steel does not rust because :
A)
chromium and nickel combine with iron
done
clear
B)
chromium forms an oxide layer and protects iron from rusting
done
clear
C)
nickel present in it, does not rust
done
clear
D)
iron forms a hard chemical compound with chromium present in it
done
clear
View Answer play_arrow
question_answer 65) Which of the following combinations can be used to synthesise ethanol?
A)
\[C{{H}_{3}}MgI\] and \[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}MgI\] and \[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[C{{H}_{3}}MgI\] and \[CH{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[C{{H}_{3}}MgI\] and \[HCOO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 66) A solution contains \[1.2046\times {{10}^{-24}}\] hydrochloric acid molecules in one \[d{{m}^{3}}\] of the solution. The strength of the solution is :
A)
6 N
done
clear
B)
2 N
done
clear
C)
4 N
done
clear
D)
8 N
done
clear
View Answer play_arrow
question_answer 67) Nuclear theory of the atom was put forward by:
A)
Rutherford
done
clear
B)
Aston
done
clear
C)
Neils Bohr
done
clear
D)
J.J. Thomson
done
clear
View Answer play_arrow
question_answer 68) In acetylene molecule, the two carbon atoms are linked by :
A)
one sigma-bond and two pi-bonds
done
clear
B)
two sigma-bonds and one pi-bond
done
clear
C)
three sigma-bonds
done
clear
D)
three pi-bonds
done
clear
View Answer play_arrow
question_answer 69) The enthalpy of reaction,\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\to {{H}_{2}}O(g)\] is \[\Delta {{H}_{1}}\] and that of\[{{H}_{{{2}_{(g)}}}}+\frac{1}{2}{{O}_{2}}(g)\to {{H}_{2}}{{O}_{(l)}}\] is \[\Delta {{H}_{2}}\]. Then:
A)
\[\Delta {{H}_{1}}<\Delta {{H}_{2}}\]
done
clear
B)
\[\Delta {{H}_{1}}+\Delta {{H}_{2}}=0\]
done
clear
C)
\[\Delta {{H}_{1}}>\Delta {{H}_{2}}\]
done
clear
D)
\[\Delta {{H}_{1}}=\Delta {{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 70) A radioactive isotope decays at such a rate that after 192 minutes only 1/16 of the original amount remains :
A)
32 min
done
clear
B)
48 min
done
clear
C)
12 min
done
clear
D)
24 min
done
clear
View Answer play_arrow
question_answer 71) The pressure and temperature of \[4\text{ }d{{m}^{3}}\] of carbon dioxide gas are doubled. Then the volume of carbon dioxide gas would be :
A)
\[2\text{ }d{{m}^{3}}\]
done
clear
B)
\[3\text{ }d{{m}^{3}}\]
done
clear
C)
\[4\text{ }d{{m}^{3}}\]
done
clear
D)
\[8\text{ }d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 72) 4g of copper was dissolved in concentrated nitric acid. The copper nitrate solution on strong heating gave 5 g of its oxide. The equivalent weight of copper is:
A)
23
done
clear
B)
32
done
clear
C)
12
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 73) In the manufacture of ammonia by Habers process,\[{{N}_{2(g)}}+3{{H}_{2}}2N{{H}_{3(g)}}+92.3\,\,kJ\]which of the following conditions is unfavourable?
A)
Increasing the temperature
done
clear
B)
Increasing the pressure
done
clear
C)
Reducing the temperature
done
clear
D)
Removing ammonia as it is formed
done
clear
View Answer play_arrow
question_answer 74) The chemical equilibrium of a reversible reaction is not influenced by :
A)
pressure
done
clear
B)
catalyst
done
clear
C)
concentration of the reactants
done
clear
D)
temperature
done
clear
View Answer play_arrow
question_answer 75) Cumene process is the most important commercial method for the manufacture of phenol. Cumene is :
A)
1-methyl ethyl benzene
done
clear
B)
ethyl benzene
done
clear
C)
vinyl benzene
done
clear
D)
propyl benzene
done
clear
View Answer play_arrow
question_answer 76) The reagent which does not give acid chloride on treating with a carboxylic acid is :
A)
\[PC{{l}_{5}}\]
done
clear
B)
\[C{{l}_{2}}\]
done
clear
C)
\[SOC{{l}_{2}}\]
done
clear
D)
\[PC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) Among the halogens, the one which is oxidised by nitric acid is :
A)
fluorine
done
clear
B)
iodine
done
clear
C)
chlorine
done
clear
D)
bromine
done
clear
View Answer play_arrow
question_answer 78) The metal which does not form ammonium nitrate by reaction with dilute nitric acid is :
A)
\[Al\]
done
clear
B)
Fe
done
clear
C)
Pb
done
clear
D)
Mg
done
clear
View Answer play_arrow
question_answer 79) The elements with atomic numbers 9, 17, 35, 53, 85 are all :
A)
noble gases
done
clear
B)
halogens
done
clear
C)
heavy metals
done
clear
D)
light metals
done
clear
View Answer play_arrow
question_answer 80) In the electrolytic method of obtaining aluminium from purified bauxite, Cryolite is added to the charge in order to :
A)
minimise the heat loss due to radiation
done
clear
B)
protect aluminium produced from oxygen
done
clear
C)
dissolve bauxite and render it conductor of electricity
done
clear
D)
lower the melting point of bauxite
done
clear
View Answer play_arrow
question_answer 81) The number of 2p electrons having spin quantum number \[s=-1/2\] are :
A)
6
done
clear
B)
0
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 82) Pick out the alkane which differs from the other members of the group:
A)
2,2-dimethyl propane
done
clear
B)
pentane
done
clear
C)
2-methyl butane
done
clear
D)
2,2-dimethyl butane
done
clear
View Answer play_arrow
question_answer 83) 56 g of nitrogen and 8g of hydrogen gas are heated in a closed vessel. At equilibrium 34 g of ammonia are present. The equilibrium number of moles of nitrogen, hydrogen and ammonia are respectively:
A)
1, 2, 2
done
clear
B)
2, 2, 1
done
clear
C)
1, 1, 2
done
clear
D)
2, 1, 2
done
clear
View Answer play_arrow
question_answer 84) A process is taking place at constant temperature and pressure. Then:
A)
\[\Delta H=\Delta E\]
done
clear
B)
\[\Delta H=T\Delta S\]
done
clear
C)
\[\Delta H=0\]
done
clear
D)
\[\Delta S=0\]
done
clear
View Answer play_arrow
question_answer 85) In a galvanic cell, the electrons flow from:
A)
anode to cathode through the solution
done
clear
B)
cathode to anode through the solution
done
clear
C)
anode to cathode through the external circuit
done
clear
D)
cathode to anode through the external circuit
done
clear
View Answer play_arrow
question_answer 86) The reaction, \[2\,S{{O}_{2(g)}}+{{O}_{2(g)}}2S{{O}_{3(g)}}\] is carried out in a \[1\,\,d{{m}^{3}}\] vessel and \[2\,\,d{{m}^{3}}\] vessel separately. The ratio of the reaction velocities will be:
A)
1 : 8
done
clear
B)
1 : 4
done
clear
C)
4 : 1
done
clear
D)
8 : 1
done
clear
View Answer play_arrow
question_answer 87) In a mixture of acetic acid and sodium acetate the ratio of concentrations of the salt to the acid is increased ten times. Then the pH of the solution :
A)
increases by one
done
clear
B)
decreases by one
done
clear
C)
decreases ten fold
done
clear
D)
increases ten fold
done
clear
View Answer play_arrow
question_answer 88) When a mixture of methane and oxygen is passed through heated molybdenum oxide, the main product formed is :
A)
methanoic acid
done
clear
B)
ethanol
done
clear
C)
methanol
done
clear
D)
methanol
done
clear
View Answer play_arrow
question_answer 89) Benzene can be obtained by heating either benzoic acid with X or phenol with Y.X and Y are respectively:
A)
zinc dust and soda lime
done
clear
B)
soda lime and zinc dust
done
clear
C)
zinc dust and sodium hydroxide
done
clear
D)
soda lime and copper
done
clear
View Answer play_arrow
question_answer 90) An organic compound is boiled with alcoholic potash. The product is cooled and acidified with \[HCl\]. A white solid separates out. The starting compound may be:
A)
ethyl benzoate
done
clear
B)
ethyl formate
done
clear
C)
ethyl acetate
done
clear
D)
methyl acetate
done
clear
View Answer play_arrow
question_answer 91) A nitrogen containing organic compound gave an oily liquid on heating with bromine and potassium hydroxide solution. On shaking the product with acetic anhydride, an antipyretic drug was obtained. The reactions indicate that the starting compound is:
A)
aniline
done
clear
B)
benzamide
done
clear
C)
acetamide
done
clear
D)
nitrobenzene
done
clear
View Answer play_arrow
question_answer 92) The silver salt of a fatty acid on refluxing with an alkyl halide gives an :
A)
acid
done
clear
B)
ester
done
clear
C)
ether
done
clear
D)
amine
done
clear
View Answer play_arrow
question_answer 93) Pick out the one which does not belong to the family:
A)
pepsin
done
clear
B)
cellulose
done
clear
C)
ptyalin
done
clear
D)
lipase
done
clear
View Answer play_arrow
question_answer 94) Which one of the following is wrongly matched?
A)
Saponification of\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\] - second order reaction
done
clear
B)
Hydrolysis of\[C{{H}_{3}}COOC{{H}_{3}}\] - pseudo animalcular
done
clear
C)
Decomposition\[{{H}_{2}}{{O}_{2}}\] - first order rof reaction
done
clear
D)
Combination of \[{{H}_{2}}\]and \[B{{r}_{2}}\] to give HBr - first order reaction
done
clear
View Answer play_arrow
question_answer 95) The diameter of colloidal particles range from:
A)
\[{{10}^{-6}}\,m\] to \[{{10}^{-9}}\,m\]
done
clear
B)
\[{{10}^{-9}}\,m\] to \[{{10}^{-12}}\,m\]
done
clear
C)
\[{{10}^{-3}}\,m\] to \[{{10}^{-3}}\,m\]
done
clear
D)
\[{{10}^{-3}}\,m\] to \[{{10}^{-6}}\,m\]
done
clear
View Answer play_arrow
question_answer 96) On treating a mixture of two alkyl halides with sodium metal in dry ether, 2-methyl propane was obtained. The alkyl halides are :
A)
2-chloropropane and chloromethane
done
clear
B)
2-chloropropane and chloroethane
done
clear
C)
chloromethane and chloroethane
done
clear
D)
chloromethane and 1-chloropropane
done
clear
View Answer play_arrow
question_answer 97) Which of the following statements about benzyl chloride is incorrect?
A)
It is less reactive than alkyl halides
done
clear
B)
It can be oxidised to benzaldehyde by boiling with copper nitrate solution
done
clear
C)
It is a lachrymatory liquid and answers Beilsteins test
done
clear
D)
It gives a white precipitate with alcoholic silver nitrate
done
clear
View Answer play_arrow
question_answer 98) The main product obtained when a solution of sodium carbonate reacts with mercuric chloride is :
A)
\[Hg{{(OH)}_{2}}\]
done
clear
B)
\[HgC{{O}_{3}}\,.\,HgO\]
done
clear
C)
\[HgC{{O}_{3}}\]
done
clear
D)
\[HgC{{O}_{3}}\,.\,Hg{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) In the electrothermal process, the compound displaced by silica from calcium phosphate is:
A)
calcium phosphide
done
clear
B)
phosphine
done
clear
C)
phosphorus
done
clear
D)
phosphorus pentoxide
done
clear
View Answer play_arrow
question_answer 100) The enthalpy of combustion of methane at \[{{25}^{o}}C\] is 890 kJ. The heat liberated when 3.2 g of methane is burnt is air is :
A)
445 kJ
done
clear
B)
278 kJ
done
clear
C)
-890 kJ
done
clear
D)
178 kJ
done
clear
View Answer play_arrow
question_answer 101) The velocity constant of a reaction at 290 K was found to be \[3.2\times {{10}^{-3}}\,\,{{s}^{-1}}\]. When the temperature is raised to 310 K, it will be about:
A)
\[6.4\times {{10}^{-3}}\]
done
clear
B)
\[3.2\times {{10}^{-4}}\]
done
clear
C)
\[9.6\times {{10}^{-3}}\]
done
clear
D)
\[1.28\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 102) Select the \[p{{K}_{a}}\] value of the strongest acid from the following:
A)
1.0
done
clear
B)
3.0
done
clear
C)
2.0
done
clear
D)
4.5
done
clear
View Answer play_arrow
question_answer 103) Pick out the unsaturated fatty acid from the following:
A)
stearic acid
done
clear
B)
lauric acid
done
clear
C)
oleic acid
done
clear
D)
palmitic acid
done
clear
View Answer play_arrow
question_answer 104) Nylon is not a:
A)
condensation polymer
done
clear
B)
polyamide
done
clear
C)
copolymer
done
clear
D)
homopolymer
done
clear
View Answer play_arrow
question_answer 105) The coal tar fraction which contains phenol is:
A)
middle oil
done
clear
B)
green oil
done
clear
C)
heavy oil
done
clear
D)
light oil
done
clear
View Answer play_arrow
question_answer 106) The compounds A and B are mixed in equimolar proportion to form the products,\[A+BC+D\]. At equilibrium, one third of A and B are consumed. The equilibrium constant for die reaction is:
A)
0.5
done
clear
B)
4.0
done
clear
C)
2.5
done
clear
D)
0.25
done
clear
View Answer play_arrow
question_answer 107) In froth floatation process for the purification of ores, the particles of ore float because:
A)
their surface is not easily wetted by water
done
clear
B)
they are light
done
clear
C)
they are insoluble
done
clear
D)
they bear electrostatic charge
done
clear
View Answer play_arrow
question_answer 108) Which of the following statements about amorphous solids is incorrect?
A)
They melt over a range of temperature
done
clear
B)
They are anisotropic
done
clear
C)
There is no orderly arrangement of particles
done
clear
D)
They are rigid and incompressible
done
clear
View Answer play_arrow
question_answer 109) Hydrogen diffuses six times faster than gas A. The molar mass of gas A is:
A)
72
done
clear
B)
6
done
clear
C)
24
done
clear
D)
36
done
clear
View Answer play_arrow
question_answer 110) Dulong and Petits law is valid only for:
A)
metals
done
clear
B)
non-metals
done
clear
C)
gaseous elements
done
clear
D)
solid elements
done
clear
View Answer play_arrow
question_answer 111) Identify the gas which is readily adsorbed by activated charcoal:
A)
\[{{N}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 112) If the distance between \[N{{a}^{+}}\] and \[C{{l}^{-}}\] ions in sodium chloride crystal is X pm, the length of the edge of the unit cell is :
A)
4 X pm
done
clear
B)
X/4 pm
done
clear
C)
X/2 pm
done
clear
D)
2 X pm
done
clear
View Answer play_arrow
question_answer 113) Which of the following statements is incorrect?
A)
In \[{{K}_{3}}\,[Fe{{(CN)}_{6}}]\], the ligand has satisfied only the secondary valency of ferric ion
done
clear
B)
In \[{{K}_{3}}\,[Fe{{(CN)}_{6}}]\], the ligand has satisfied both primary and secondary valancies of ferric ion
done
clear
C)
In \[{{K}_{4}}\,[Fe{{(CN)}_{6}}]\] the ligand has satisfied both primary and secondary valancies of ferrous ion
done
clear
D)
In \[[Cu{{(N{{H}_{3}})}_{4}}]S{{O}_{4}}\] the ligand has satisfied only the secondary valency of copper
done
clear
View Answer play_arrow
question_answer 114) 2-acetoxy benzoic acid is used as an:
A)
antimalarial
done
clear
B)
antidepressant
done
clear
C)
antiseptic
done
clear
D)
antipyretic
done
clear
View Answer play_arrow
question_answer 115) A nucleoside on hydrolysis gives :
A)
a heterocyclic base and orthophosphoric acid
done
clear
B)
an aldopentose, a heterocyclic base and orthophosphoric acid
done
clear
C)
an aldopentose and a heterocyclic base
done
clear
D)
an aldopentose and orthophosphoric acid
done
clear
View Answer play_arrow
question_answer 116) In qualitative analysis, in order to detect second group basic radical, \[{{H}_{2}}S\] gas is passed
A)
in the presence of dilute \[HCl\] to:
done
clear
B)
increase the dissociation of \[{{H}_{2}}S\] decrease the dissociation of salt solution
done
clear
C)
decrease the dissociation of \[{{H}_{2}}S\]
done
clear
D)
increase the dissociation of salt solution
done
clear
View Answer play_arrow
question_answer 117) Aluminium displaces hydrogen from dilute \[HCl\] whereas silver does not. The e.m.f. of a cell prepared by combining \[Al/A{{l}^{3+}}\] and \[Ag/A{{g}^{+}}\] is 2.46V. The reduction potential of silver electrode is + 0.80 V. The reduction potential of aluminium electrode is :
A)
+ 1.66 V
done
clear
B)
-3.26 V
done
clear
C)
3.26 V
done
clear
D)
-1.66 V
done
clear
View Answer play_arrow
question_answer 118) The first fraction obtained during the fractionation of petroleum is :
A)
hydrocarbon gases
done
clear
B)
kerosene oil
done
clear
C)
gasoline
done
clear
D)
diesel oil
done
clear
View Answer play_arrow
question_answer 119) Which of the following compounds gives trichloromethane on distilling with bleaching powder?
A)
Methanal
done
clear
B)
Phenol
done
clear
C)
Ethanol
done
clear
D)
Methanol
done
clear
View Answer play_arrow
question_answer 120) Benzoin is:
A)
compound containing an aldehyde and a ketonic group
done
clear
B)
\[\alpha \], P-unsaturated acid
done
clear
C)
\[\alpha \]-hydroxy aldehyde
done
clear
D)
\[\alpha \]-hydroxy ketone
done
clear
View Answer play_arrow
question_answer 121) \[\sim p\wedge q\] is logically equivalent to :
A)
\[p\to q\]
done
clear
B)
\[q\to p\]
done
clear
C)
\[\sim (p\to q)\]
done
clear
D)
\[\sim (q\to p)\]
done
clear
View Answer play_arrow
question_answer 122) Which of the following is the inverse of the proposition: If a number is a prime then it is odd?
A)
If a number is not a prime then it is odd
done
clear
B)
If a number is not a prime then it is not odd
done
clear
C)
If a number is not odd then it is not a prime
done
clear
D)
If a number is odd then it is a prime
done
clear
View Answer play_arrow
question_answer 123) What must be the matrix X if\[2\,X+\left[ \begin{matrix} 1 & 2 \\ 3 & 4 \\ \end{matrix} \right]=\left[ \begin{matrix} 3 & 8 \\ 7 & 2 \\ \end{matrix} \right]\,\,?\]
A)
\[\left[ \begin{matrix} 1 & 3 \\ 2 & -1 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 1 & -3 \\ 2 & -1 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 2 & 6 \\ 4 & -2 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 2 & -6 \\ 4 & -2 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 124) The value of be \[\left| \begin{matrix} 1 & 1 & 1 \\ bc & ca & ab \\ b+c & c+a & a+b \\ \end{matrix} \right|\] is :
A)
1
done
clear
B)
0
done
clear
C)
\[(a-b)(b-c)(c-a)\]
done
clear
D)
\[(a+b)(b+c)(c+a)\]
done
clear
View Answer play_arrow
question_answer 125) The value of \[\left| \begin{matrix} 441 & 442 & 443 \\ 445 & 446 & 447 \\ 449 & 450 & 451 \\ \end{matrix} \right|\] is :
A)
\[441\times 446\times 4510\]
done
clear
B)
0
done
clear
C)
-1
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 126) \[(\vec{a}.\,\hat{i})\,\hat{i}+(\vec{a}\,.\,\hat{j})\,\hat{j}+\,(\vec{a}\,.\,\hat{k})\,\hat{k}\] is equal to :
A)
\[\vec{a}\]
done
clear
B)
\[2\,\vec{a}\]
done
clear
C)
\[3\,\vec{a}\]
done
clear
D)
\[\vec{0}\]
done
clear
View Answer play_arrow
question_answer 127) Inverse of the matrix \[\left[ \begin{matrix} \cos 2\theta & -\sin 2\theta \\ \sin 2\theta & \cos 2\theta \\ \end{matrix} \right]\] is :
A)
\[\left[ \begin{matrix} \cos 2\theta & -\sin 2\theta \\ \sin 2\theta & \cos 2\theta \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} \cos 2\theta & \sin 2\theta \\ \sin 2\theta & -\cos 2\theta \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} \cos 2\theta & \sin 2\theta \\ \sin 2\theta & \cos 2\theta \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} \cos 2\theta & \sin 2\theta \\ -\sin 2\theta & \cos 2\theta \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 128) If \[|\overrightarrow{a}|=3,\,\,|\overrightarrow{b}|=4\], then a value of \[\lambda \] for which\[\overrightarrow{a}+\lambda ,\overrightarrow{b}\] is perpendicular to \[\overrightarrow{a}-\lambda ,\overrightarrow{b}\] is :
A)
\[\frac{9}{16}\]
done
clear
B)
\[\frac{3}{4}\]
done
clear
C)
\[\frac{3}{2}\]
done
clear
D)
\[\frac{3}{2}\]
done
clear
View Answer play_arrow
question_answer 129) The projection of \[\vec{a}=2\,\hat{i}+3\,\hat{j}-2\hat{k}\] on\[\vec{b}=\,\hat{i}+2\,\hat{j}+3\hat{k}\] is :
A)
\[\frac{1}{\sqrt{14}}\]
done
clear
B)
\[\frac{2}{\sqrt{14}}\]
done
clear
C)
\[\sqrt{14}\]
done
clear
D)
\[\frac{-2}{\sqrt{14}}\]
done
clear
View Answer play_arrow
question_answer 130) In the group ( 1, 2, 3, 4, 5, 6) under multiplication modulo \[7,{{2}^{-1}}\times 4\] is equal to :
A)
1
done
clear
B)
4
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 131) The maximum of the function \[3\cos x-4\sin x\] is :
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 132) If the distance s metres traversed by a particle in t seconds is given by \[s={{t}^{3}}-3{{t}^{2}}\], then the velocity of the particle when the acceleration is zero, in metre/sec, is :
A)
3
done
clear
B)
- 2
done
clear
C)
- 3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 133) For the curve \[{{y}^{n}}={{a}^{n-1}}\] if the subnormal at any point is a constant then n is equal to :
A)
1
done
clear
B)
2
done
clear
C)
- 2
done
clear
D)
- 1
done
clear
View Answer play_arrow
question_answer 134) If \[x=A\cos 4t+B\sin 4t\] then \[\frac{{{d}^{2}}x}{d{{t}^{2}}}\] is equal to :
A)
\[-16\text{ }x\]
done
clear
B)
\[16x\]
done
clear
C)
\[x\]
done
clear
D)
\[-x\]
done
clear
View Answer play_arrow
question_answer 135) If tangent to the curve \[x=a{{t}^{2}},y=2at\] is perpendicular to x-axis, then its point of contact is :
A)
\[(a,\,a)\]
done
clear
B)
\[(0,\,a)\]
done
clear
C)
\[(0,0)\]
done
clear
D)
\[(0,0)\]
done
clear
View Answer play_arrow
question_answer 136) The general solution of the differential equation \[\frac{dy}{dx}+\frac{1+\cos 2y}{1-\cos 2y}=0\] is given by :
A)
\[\tan y+\cot x=c\]
done
clear
B)
\[\tan y-\cot x=c\]
done
clear
C)
\[\tan x-\cot y=c\]
done
clear
D)
\[\tan x+\cot x=c\]
done
clear
View Answer play_arrow
question_answer 137) The degree of the differential equation\[{{\left( 1+{{\left( \frac{dy}{dx} \right)}^{2}} \right)}^{3/4}}={{\left( \frac{{{d}^{2}}y}{d{{x}^{2}}} \right)}^{1/3}}\] is:
A)
2
done
clear
B)
4
done
clear
C)
9
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 138) The area enclosed between the curves \[y={{x}^{3}}\] and \[y=\sqrt{x}\] is, (in square units) :
A)
\[\frac{5}{3}\]
done
clear
B)
\[\frac{5}{4}\]
done
clear
C)
\[\frac{5}{12}\]
done
clear
D)
\[\frac{12}{5}\]
done
clear
View Answer play_arrow
question_answer 139) \[\int_{0}^{\pi /8}{{{\cos }^{3}}4\,\theta \,d\,\theta }\] is equal to :
A)
\[\frac{5}{3}\]
done
clear
B)
\[\frac{5}{4}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[\frac{1}{6}\]
done
clear
View Answer play_arrow
question_answer 140) \[\int_{0}^{\pi /2}{\frac{\cos x-\sin x}{1+\cos x\sin x}dx}\] is equal to :
A)
0
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{4}\]
done
clear
D)
\[\frac{\pi }{6}\]
done
clear
View Answer play_arrow
question_answer 141) If \[a{{x}^{2}}-{{y}^{2}}+4x-y=0\] represents a pair of lines, then a is equal to :
A)
- 16
done
clear
B)
16
done
clear
C)
4
done
clear
D)
- 4
done
clear
View Answer play_arrow
question_answer 142) What is the equation of the locus of a point which moves such that 4 times its distance from the x-axis is the square of its distance from the origin?
A)
\[{{x}^{2}}+{{y}^{2}}-4y=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}-4|y|=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}-4x=0\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}-4|x|=0\]
done
clear
View Answer play_arrow
question_answer 143) Equation of the straight line making equal intercepts on the axes and passing through the point (2, 4) is :
A)
\[4x-y-4=0\]
done
clear
B)
\[2x+y-8=0\]
done
clear
C)
\[x+y-6=0\]
done
clear
D)
\[x+2y-10=0\]
done
clear
View Answer play_arrow
question_answer 144) If the area of the triangle with vertices \[(x,\text{ }0),\text{ (}1,1\text{)}\] and \[(0,2)\] is 4 square units, then the value of x is :
A)
-2
done
clear
B)
-4
done
clear
C)
- 6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 145) \[\underset{x\to \frac{\pi }{2}}{\mathop{\lim }}\,\frac{\frac{\pi }{2}-\theta }{\cot \theta }\]
A)
0
done
clear
B)
- 1
done
clear
C)
1
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 146) The co-axial system of circles given by\[{{x}^{2}}+{{y}^{2}}+2gx+c=0\] for \[c<0\] represents :
A)
intersecting circles
done
clear
B)
non intersecting circles
done
clear
C)
touching circles
done
clear
D)
touching or non-intersecting circles
done
clear
View Answer play_arrow
question_answer 147) The radius of the circle passing through the point (6, 2) and two of whose diameters are \[x+y=6\] and \[x+2y=4\] is :
A)
4
done
clear
B)
6
done
clear
C)
20
done
clear
D)
\[\sqrt{20}\]
done
clear
View Answer play_arrow
question_answer 148) If (0, 6) and (0, 3) arc respectively the vertex and focus of a parabola, then its equation is :
A)
\[{{x}^{2}}+12y=72\]
done
clear
B)
\[{{x}^{2}}-12y=72\]
done
clear
C)
\[{{y}^{2}}-12x=72\]
done
clear
D)
\[{{y}^{2}}+12x=72\]
done
clear
View Answer play_arrow
question_answer 149) For the ellipse\[24{{x}^{2}}+9{{y}^{2}}-150x-90y+225=0\] the eccentricity e is equal to :
A)
\[\frac{2}{5}\]
done
clear
B)
\[\frac{3}{5}\]
done
clear
C)
\[\frac{4}{5}\]
done
clear
D)
\[\frac{1}{5}\]
done
clear
View Answer play_arrow
question_answer 150) If the foci of the ellipse \[\frac{{{x}^{2}}}{16}+\frac{{{y}^{2}}}{{{b}^{2}}}=1\] and the hyperbola \[\frac{{{x}^{2}}}{144}-\frac{{{y}^{2}}}{81}=\frac{1}{25}\] coincide, then the value of \[{{b}^{2}}\] is :
A)
1
done
clear
B)
7
done
clear
C)
5
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 151) The differential coefficient is \[f(\sin x)\] with respect to \[x\] where \[f(x)=\log \,x\] is :
A)
\[\tan x\]
done
clear
B)
\[\cot x\]
done
clear
C)
\[f(\cos x)\]
done
clear
D)
\[\frac{1}{x}\]
done
clear
View Answer play_arrow
question_answer 152) If \[f(x)=\left\{ \frac{1-\cos x}{\begin{align} & x\,\,x=0 \\ & k \\ \end{align}}x\ne 0 \right.\] is continuous at\[x=0\], then the value of k is :
A)
0
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[-\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 153) If \[\omega =\frac{-1+\sqrt{3}i}{2}\] then \[{{(3+\omega +3{{\omega }^{2}})}^{4}}\] is :
A)
16
done
clear
B)
- 16
done
clear
C)
\[16\,\omega \]
done
clear
D)
\[16\,{{\omega }^{2}}\]
done
clear
View Answer play_arrow
question_answer 154) If \[y={{\tan }^{-1}}(\sec \,x-\tan x)\], then \[\frac{d\,y}{d\,x}\] is equal to:
A)
2
done
clear
B)
-2
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[\frac{d\,y}{d\,x}\]
done
clear
View Answer play_arrow
question_answer 155) If \[x+\frac{1}{x}=2\cos \alpha \] then \[{{x}^{n}}+\frac{1}{{{x}^{n}}}\] is equal to :
A)
\[{{2}^{n}}\cos \alpha \]
done
clear
B)
\[{{2}^{n}}\cos n\alpha \]
done
clear
C)
\[2i\sin n\alpha \]
done
clear
D)
\[2\cos \,n\alpha \]
done
clear
View Answer play_arrow
question_answer 156) \[\int_{-1}^{1}{\,|1-x|\,dx}\] is equal to :
A)
-2
done
clear
B)
0
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 157) \[\int{\frac{d\,x}{x\,({{x}^{7}}+1)}}\] is equal to :
A)
\[\log \left( \frac{{{x}^{7}}}{{{x}^{7}}+1} \right)+c\]
done
clear
B)
\[\frac{1}{7}\log \left( \frac{{{x}^{7}}}{{{x}^{7}}+1} \right)+c\]
done
clear
C)
\[\log \left( \frac{{{x}^{7}}+1}{{{x}^{7}}} \right)+c\]
done
clear
D)
\[\frac{1}{7}\log \left( \frac{{{x}^{7}}+1}{{{x}^{7}}} \right)+c\]
done
clear
View Answer play_arrow
question_answer 158) \[\int{\sqrt{x}\,{{e}^{\sqrt{x}}}}dx\] is equal to :
A)
\[2\sqrt{x}\,-{{e}^{\sqrt{x}}}-4\sqrt{x{{e}^{\sqrt{x}}}}+c\]
done
clear
B)
\[(2x-4\sqrt{x}\,+4){{e}^{\sqrt{x}}}+c\]
done
clear
C)
\[(2x+4\sqrt{x}\,+4){{e}^{\sqrt{x}}}+c\]
done
clear
D)
\[(1-4\sqrt{x}){{e}^{\sqrt{x}}}+c\]
done
clear
View Answer play_arrow
question_answer 159) \[\int{\frac{d\,x}{{{x}^{2}}+2x+2}}\] is equal to :
A)
\[{{\sin }^{-1}}(x+1)+c\]
done
clear
B)
\[\sin \,\,{{h}^{-1}}(x+1)+c\]
done
clear
C)
\[\tan \,\,{{h}^{-1}}(x+1)+c\]
done
clear
D)
\[{{\tan }^{-1}}(x+1)+c\]
done
clear
View Answer play_arrow
question_answer 160) If a tangent to the curve \[y=6x-{{x}^{2}}\] is parallel to the line \[4x-2y-1=0\], then the point of tangency on the curve is :
A)
(2, 8)
done
clear
B)
(8, 2)
done
clear
C)
(6, 1)
done
clear
D)
(4, 2)
done
clear
View Answer play_arrow
question_answer 161) 0.5737373 ... is equal to :
A)
\[\frac{284}{497}\]
done
clear
B)
\[\frac{284}{495}\]
done
clear
C)
\[\frac{568}{999}\]
done
clear
D)
\[\frac{567}{990}\]
done
clear
View Answer play_arrow
question_answer 162) The number of solutions for the equation\[{{x}^{2}}-5\,|x|+6=0\] is :
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 163) How many numbers of 6 digits can be formed from the digits of the number 112233?
A)
30
done
clear
B)
60
done
clear
C)
90
done
clear
D)
120
done
clear
View Answer play_arrow
question_answer 164) The last digit in 7300 is :
A)
7
done
clear
B)
9
done
clear
C)
1
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 165) If \[\frac{\log x}{a-b}=\frac{\log y}{b-c}=\frac{\log z}{c-a}\], then \[xyz\] is equal to :
A)
0
done
clear
B)
1
done
clear
C)
-1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 166) The smallest positive integer n for which\[{{(1+i)}^{2n}}={{(1-i)}^{2n}}\] is :
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 167) If \[{{\cos }^{-1}}p+{{\cos }^{-1}}q+{{\cos }^{-1}}r=\pi \] then\[{{p}^{2}}+{{q}^{2}}+{{r}^{2}}+2pqr\] is equal to :
A)
3
done
clear
B)
1
done
clear
C)
2
done
clear
D)
- 1
done
clear
View Answer play_arrow
question_answer 168) If \[{{\sin }^{-1}}\frac{x}{5}+\cos e{{c}^{-1}}\frac{5}{4}=\frac{\pi }{2}\], then \[x\] is equal to :
A)
1
done
clear
B)
4
done
clear
C)
3
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 169) If \[0\le x\le \pi \] and \[{{81}^{{{\sin }^{2}}x}}+{{81}^{{{\cos }^{2}}x}}=30\], then \[x\] is equal to :
A)
\[\frac{\pi }{6}\]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{4}\]
done
clear
D)
\[\frac{3\pi }{4}\]
done
clear
View Answer play_arrow
question_answer 170) The equation of the director circle of the hyperbola \[\frac{{{x}^{2}}}{16}-\frac{{{y}^{2}}}{4}=1\] is given by :
A)
\[{{x}^{2}}+{{y}^{2}}=16\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}=4\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}=20\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}=12\]
done
clear
View Answer play_arrow
question_answer 171) If \[{{Q}_{1}}\] is the set of all relations other than 1 with the binary operation * defined by \[a\,*b=a+b-ab\] for all a, b in \[{{Q}_{1}}\], then the identity in \[{{Q}_{1}}\] with respect to * is :
A)
1
done
clear
B)
0
done
clear
C)
-1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 172) The circle \[{{x}^{2}}+{{y}^{2}}-8x+4y+4=0\] touches :
A)
x-axis
done
clear
B)
y-axis
done
clear
C)
both axis
done
clear
D)
neither .y-axis nor y-axis
done
clear
View Answer play_arrow
question_answer 173) Which of the following is true?
A)
The set of all fourth roots of unity is a multiplicative group
done
clear
B)
The set of all cube roots of unity is an additive group
done
clear
C)
\[{{(ab)}^{-1}}={{a}^{-1}}{{b}^{-1}}\] for all a, b in any group G
done
clear
D)
If \[{{(ab)}^{2}}={{a}^{2}}{{b}^{2}}\] for all a, b in any group G, then the group G is non abelian
done
clear
View Answer play_arrow
question_answer 174) The set of all integral multiples of 5 is a subgroup of :
A)
The set of all rational numbers under multiplication
done
clear
B)
The set of all integers under multiplication
done
clear
C)
The set of all non zero rational numbers under multiplication
done
clear
D)
The set of all integers under addition
done
clear
View Answer play_arrow
question_answer 175) The value of \[k\] so that \[{{x}^{2}}+{{y}^{2}}+kx+4y+2=0\] and\[2({{x}^{2}}+{{y}^{2}})-4x-3y+k=0\] cut orthogonally is :
A)
\[\frac{10}{3}\]
done
clear
B)
\[-\frac{8}{3}\]
done
clear
C)
\[-\frac{10}{3}\]
done
clear
D)
\[\frac{8}{3}\]
done
clear
View Answer play_arrow
question_answer 176) \[\underset{x\to \infty }{\mathop{\lim }}\,{{\left( 1-\frac{4}{x-1} \right)}^{3x-1}}\] is equal to :
A)
\[{{e}^{12}}\]
done
clear
B)
\[{{e}^{-12}}\]
done
clear
C)
\[{{e}^{4}}\]
done
clear
D)
\[{{e}^{3}}\]
done
clear
View Answer play_arrow
question_answer 177) If \[A+B+C={{180}^{o}}\] then \[\sum \tan \frac{A}{2}\tan \frac{B}{2}\] is equal to :
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 178) In a triangle ABC if \[b=2,B={{30}^{o}}\] then the area of the circumcircle of triangle ABC in square units is :
A)
\[\pi \]
done
clear
B)
\[2\,\pi \]
done
clear
C)
\[4\,\pi \]
done
clear
D)
\[6\,\pi \]
done
clear
View Answer play_arrow
question_answer 179) If \[\sin x+{{\sin }^{2}}x=1\], then\[{{\cos }^{12}}x+3{{\cos }^{10}}x+3{{\cos }^{8}}x+{{\cos }^{6}}x\] is equal to:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 180) If R denotes the set of all real number, then the function \[f:R\to R\] defined \[f(x)=|x|\] is :
A)
one-one only
done
clear
B)
onto only
done
clear
C)
both one-one and onto
done
clear
D)
neither one-one nor onto
done
clear
View Answer play_arrow