question_answer 1) The dimensions of \[\frac{a}{b}\] in the equation\[P=\frac{a-{{t}^{2}}}{bx}\]where P is pressure, \[x\] is distance and t is time, are:
A)
\[\left[ {{M}^{2}}L{{T}^{-3}} \right]\]
done
clear
B)
\[\left[ M{{T}^{-2}} \right]\]
done
clear
C)
\[\left[ L{{T}^{-3}} \right]\]
done
clear
D)
\[\left[ M{{L}^{3}}{{T}^{-1}} \right]\]
done
clear
View Answer play_arrow
question_answer 2) Three vectors satisfy the relation \[\overrightarrow{A.}\overrightarrow{B}=0\] and \[\overrightarrow{A.}\overrightarrow{C}=0,\]then A is parallel to:
A)
\[\overrightarrow{C}\]
done
clear
B)
\[\overrightarrow{B}\]
done
clear
C)
\[\overrightarrow{B}\times \overrightarrow{C}\]
done
clear
D)
\[\overrightarrow{B}.\overrightarrow{C}\]
done
clear
View Answer play_arrow
question_answer 3) A student is standing at a distance of 50 metre from the bus. As soon as the bus begins its motion with an acceleration of \[1\text{ }m{{s}^{-2}}\], the student starts running towards the bus with a uniform velocity u. Assuming the motion to be along a straight road, the minimum value of n, so that the student is able to catch the bus is:
A)
\[8\text{ }m{{s}^{-1}}\]
done
clear
B)
\[5\text{ }m{{s}^{-1}}\]
done
clear
C)
\[12\text{ }m{{s}^{-1}}\]
done
clear
D)
\[10\text{ }m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 4) For a given velocity, a projectile has the same range R for two angles of projection if \[{{t}_{1}}\] and \[{{t}_{2}}\] are the time of flight in the two cases, then:
A)
\[{{t}_{1}}{{t}_{2}}\propto R\]
done
clear
B)
\[{{t}_{1}}{{t}_{2}}\propto {{R}^{2}}\]
done
clear
C)
\[{{t}_{1}}{{t}_{2}}\propto \frac{1}{{{R}^{2}}}\]
done
clear
D)
\[{{t}_{1}}{{t}_{2}}\propto \frac{1}{R}\]
done
clear
View Answer play_arrow
question_answer 5) Weight of a body of mass m decreases by 1% when it is raised to height h above the earths surface. If the body is taken to a depth h in a mine, change in its weight is:
A)
0.5% decrease
done
clear
B)
2% decrease
done
clear
C)
0.5% increase
done
clear
D)
1% increase
done
clear
View Answer play_arrow
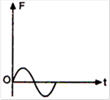
question_answer 6)
The displacement time graph of a particle executing S.H.M. is as shown in the figure.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 7) Which of the following sets of concurrent forces maybe in equilibrium?
A)
\[{{F}_{1}}=3N,{{F}_{2}}=5N,\,{{F}_{3}}=1N\]
done
clear
B)
(b \[{{F}_{1}}=3N,{{F}_{2}}=5N,\,{{F}_{3}}=9N\]
done
clear
C)
\[{{F}_{1}}=3N,{{F}_{2}}=5N,\,{{F}_{1}}=6N\]
done
clear
D)
\[{{F}_{1}}=3N,{{F}_{2}}=5N,\,{{F}_{3}}=15N\]
done
clear
View Answer play_arrow
question_answer 8) Youngs modulus of perfectly rigid body material is:
A)
infinite
done
clear
B)
zero
done
clear
C)
\[10\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
D)
\[1\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) An ideal monoatomic gas at \[{{27}^{o}}C\] is compressed adiabatically to 8/27 times of its present volume. The increase in temperature of the gas is:
A)
\[{{375}^{o}}C\]
done
clear
B)
\[{{402}^{o}}C\]
done
clear
C)
\[{{175}^{o}}C\]
done
clear
D)
\[{{475}^{o}}C\]
done
clear
View Answer play_arrow
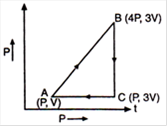
question_answer 10)
A sample of ideal monoatomic gas is taken round the cycle ABCA as shown in the figure. The work done during the cycle is:
A)
3 PV
done
clear
B)
zero
done
clear
C)
9 PV
done
clear
D)
6 PV
done
clear
View Answer play_arrow
question_answer 11) A bucket full of hot water is kept in a room. It cools from \[{{75}^{o}}C\] to \[{{70}^{o}}C\] in \[{{t}_{1}}\] minutes, from \[{{70}^{o}}C\] to \[{{65}^{o}}C\] in \[{{t}_{2}}\] minutes and from \[{{65}^{o}}C\]to \[{{60}^{o}}C\] in \[{{t}_{3}}\] minutes. Then:
A)
\[{{t}_{1}}<{{t}_{2}}<{{t}_{3}}\]
done
clear
B)
\[{{t}_{1}}={{t}_{2}}={{t}_{3}}\]
done
clear
C)
\[{{t}_{1}}<{{t}_{2}}>{{t}_{3}}\]
done
clear
D)
\[{{t}_{1}}>{{t}_{2}}>{{t}_{3}}\]
done
clear
View Answer play_arrow
question_answer 12) A fish, looking up through the water sees the outside world contained in a circular horizon. If the refractive index of water is 4/3 and the fish is 12 cm below the surface of water, the radius of the circle in centimetre is:
A)
\[\frac{12\times 3}{\sqrt{5}}\]
done
clear
B)
\[12\times 3\times \sqrt{5}\]
done
clear
C)
\[\frac{12\times 3}{\sqrt{7}}\]
done
clear
D)
\[12\times 3\times \sqrt{7}\]
done
clear
View Answer play_arrow
question_answer 13)
A given ray of light suffers minimum deviation in an equilateral prism P. Additional prisms Q and R of identical
A)
same deviation
done
clear
B)
greater deviation
done
clear
C)
total internal reflection
done
clear
D)
no deviation
done
clear
View Answer play_arrow
question_answer 14) The aperture of the objective lens of a telescope is made large so as to:
A)
increase the resolving power of the telescope
done
clear
B)
increase the magnifying power of the telescope
done
clear
C)
to focus on distant objects
done
clear
D)
make image aberrationless
done
clear
View Answer play_arrow
question_answer 15) A lamp hanging 4 metres above the table is lowered by 1 metre. The illumination on the table:
A)
decreased by 25%
done
clear
B)
increased by 25%
done
clear
C)
decreased by 66.7%
done
clear
D)
increased by 77.7%
done
clear
View Answer play_arrow
question_answer 16) The equation of a transverse wave travelling along positive \[x\] axis with amplitude 0.2 m, velocity 360 m/sec and wavelength 60 m can be written as:
A)
\[y=0.2\sin \pi \left[ 6t+\frac{x}{60} \right]\]
done
clear
B)
\[y=0.2\sin \pi \left[ 6t-\frac{x}{60} \right]\]
done
clear
C)
\[y=0.2\sin 2\pi \left[ 6t-\frac{x}{60} \right]\]
done
clear
D)
\[y=0.2\sin 2\pi \left[ 6t+\frac{x}{60} \right]\]
done
clear
View Answer play_arrow
question_answer 17) If \[{{\upsilon }_{m}}\], is the velocity of sound in moist air, \[{{\upsilon }_{d}}\]is the velocity of sound in dry air, under identical conditions of pressure and temperature:
A)
\[{{\upsilon }_{d}}<{{\upsilon }_{d}}\]
done
clear
B)
\[{{\upsilon }_{m}}>{{\upsilon }_{d}}\]
done
clear
C)
\[{{\upsilon }_{m}}\,{{\upsilon }_{d}}=1\]
done
clear
D)
\[{{\upsilon }_{m}}={{\upsilon }_{d}}\]
done
clear
View Answer play_arrow
question_answer 18) If T is the reverberation time of an auditorium of volume V, then
A)
\[T\propto {{V}^{2}}\]
done
clear
B)
\[T\propto V\]
done
clear
C)
\[T\propto \frac{1}{V}\]
done
clear
D)
\[T\propto \frac{1}{{{V}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 19) Two wires are fixed in a sonometer. Their tensions are in the ratio 8 : 1. The lengths are in the ratio 36 : 35. The diameters are in the ratio 4 : 1. Densities of the materials are in the ratio 1 : 2. If the higher frequency in the setting is 360 Hz, the beat frequency when the two wires are sounded together, is:
A)
8
done
clear
B)
5
done
clear
C)
10
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 20) A sound source is moving towards stationary listener with \[\frac{1}{10}\]th of the speed of sound. The ratio of apparent to real frequency is:
A)
\[{{\left( \frac{9}{10} \right)}^{2}}\]
done
clear
B)
\[\frac{10}{9}\]
done
clear
C)
\[\frac{11}{10}\]
done
clear
D)
\[{{\left( \frac{11}{10} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 21) If v is the speed of sound in air then the shortest length of the closed pipe which resonates to a frequency n, is:
A)
\[\frac{v}{2n}\]
done
clear
B)
\[\frac{v}{4n}\]
done
clear
C)
\[\frac{4n}{v}\]
done
clear
D)
\[\frac{2n}{v}\]
done
clear
View Answer play_arrow
question_answer 22) Cavitation is a special application property exhibited only by:
A)
ultrasonics
done
clear
B)
electromagnetic waves
done
clear
C)
audible sound
done
clear
D)
infrasonics
done
clear
View Answer play_arrow
question_answer 23) In Youngs double slit experiment, the fringe width is \[\beta \], If the entire arrangement is placed in a liquid of refractive index n, the fringe width becomes:
A)
\[n\beta \]
done
clear
B)
\[\frac{\beta }{n+1}\]
done
clear
C)
\[\frac{\beta }{n-1}\]
done
clear
D)
\[\frac{\beta }{n}\]
done
clear
View Answer play_arrow
question_answer 24) Yellow light is used in single slit diffraction experiment with slit width 0.6 mm. If yellow light is replaced by X-rays, then the pattern will reveal that:
A)
no diffraction pattern
done
clear
B)
that the central maxima narrower
done
clear
C)
less number of fringes
done
clear
D)
more number of fringes
done
clear
View Answer play_arrow
question_answer 25) In an interference experiment, third bright fringe is obtained at a point on the screen with a light of 700 nm. What should be the wavelength of die light source in order to obtain 5th bright fringe at the same point?
A)
630 nm
done
clear
B)
500 nm
done
clear
C)
420 nm
done
clear
D)
750 nm
done
clear
View Answer play_arrow
question_answer 26) If a ray of light in a denser medium enters into a rarer medium at an angle of incidence i, the angle of reflection and refraction are respectively r and r. If the reflected and refracted rays are at right angles to each other, the critical angle for the given pair of media is:
A)
\[{{\sin }^{-1}}\left( \tan r \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \tan r \right)\]
done
clear
C)
\[ta{{n}^{-1}}\left( \sin i \right)\]
done
clear
D)
\[cot\left( \tan i \right)\]
done
clear
View Answer play_arrow
question_answer 27) Waves that cannot be polarized are:
A)
electromagnetic waves
done
clear
B)
light waves
done
clear
C)
longitudinal waves
done
clear
D)
transverse waves
done
clear
View Answer play_arrow
question_answer 28) The phenomenon of rotation of plane of plane polarized light is called:
A)
Kerr effect
done
clear
B)
double refraction
done
clear
C)
optical activity
done
clear
D)
dichroism
done
clear
View Answer play_arrow
question_answer 29) As a result of interference of two coherent sources of light energy is:
A)
redistributed and the distribution does not very with time
done
clear
B)
increased
done
clear
C)
redistributed and that distribution changes with time
done
clear
D)
decreased
done
clear
View Answer play_arrow
question_answer 30) There are \[{{n}_{1}}\] photons of frequency \[{{v}_{1}}\] in a beam of light. In an equally energetic beam there are \[{{n}_{2}}\] photons of frequency \[{{v}_{2}}\]. Then the correct relation:
A)
\[\frac{{{n}_{1}}}{{{n}_{2}}}=\frac{{{v}_{1}}}{{{v}_{2}}}\]
done
clear
B)
\[\frac{{{n}_{1}}}{{{n}_{2}}}=1\]
done
clear
C)
\[\frac{{{n}_{1}}}{{{n}_{2}}}=\frac{{{v}_{2}}}{{{v}_{1}}}\]
done
clear
D)
\[\frac{{{n}_{1}}}{{{n}_{2}}}=\frac{{{v}_{2}}^{2}}{{{v}_{1}}^{2}}\]
done
clear
View Answer play_arrow
question_answer 31) A charge q is placed at the centre of the line joining two equal point charges each equal to Q. The system of 3 charges will be in equilibrium if q is equal to:
A)
\[+Q/4\]
done
clear
B)
\[-Q/2\]
done
clear
C)
\[+Q/2\]
done
clear
D)
\[-Q/4\]
done
clear
View Answer play_arrow
question_answer 32) The inward and outward electric flux from a closed surface are respectively \[8\times {{10}^{3}}\] and \[4\times {{10}^{3}}\] units. Then the net charge inside the closed surface is:
A)
\[-4\times {{10}^{3}}\] coulomb
done
clear
B)
\[4\times {{10}^{3}}\] coulomb
done
clear
C)
\[\frac{-4\times {{10}^{3}}}{{{\varepsilon }_{0}}}\] coulomb
done
clear
D)
\[-4\times {{10}^{3}}{{\varepsilon }_{0}}\]coulomb
done
clear
View Answer play_arrow
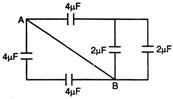
question_answer 33)
In the circuit as shown in the figure, the effective capacitance between A and B is:
A)
\[2~\mu F\]
done
clear
B)
\[2~\mu F\]
done
clear
C)
\[8~\mu F\]
done
clear
D)
\[4~\mu F\]
done
clear
View Answer play_arrow
question_answer 34) Capacitance of a parallel plate capacitor becomes \[\frac{4}{3}\] times its original value, if a dielectric slab of thickness \[t=d/2\] is inserted between the plates [d is the separation between the plates]. The dielectric constant of the slab is:
A)
4
done
clear
B)
8
done
clear
C)
2
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 35) A charged particle of mass m and charge if is released from rest in an uniform electric field E neglecting the effect of gravity, the kinetic energy of the charged particle after t second is:
A)
\[\frac{2{{E}^{2}}{{t}^{2}}}{mq}\]
done
clear
B)
\[\frac{E{{q}^{2}}m}{2{{t}^{2}}}\]
done
clear
C)
\[\frac{Eqm}{t}\]
done
clear
D)
\[\frac{{{E}^{2}}{{q}^{2}}{{t}^{2}}}{2m}\]
done
clear
View Answer play_arrow
question_answer 36) Two wires of the same dimensions but resistivities \[{{\rho }_{1}}\] and \[{{\rho }_{2}}\] are connected in series. The equivalent resistivity of the combination is:
A)
\[\frac{{{\rho }_{1}}+{{\rho }_{2}}}{2}\]
done
clear
B)
\[{{\rho }_{1}}+{{\rho }_{2}}\]
done
clear
C)
\[2\left( {{\rho }_{1}}+{{\rho }_{2}} \right)\]
done
clear
D)
\[\sqrt{{{\rho }_{1}}\,{{\rho }_{2}}}\]
done
clear
View Answer play_arrow
question_answer 37) If a 30V, 90W bulb is to be worked in 120V line, the resistance to be connected in series with the bulb is:
A)
\[20~\,\Omega \]
done
clear
B)
\[10~\,\Omega \]
done
clear
C)
\[40~\,\Omega \]
done
clear
D)
\[30~\,\Omega \]
done
clear
View Answer play_arrow
question_answer 38) The potential difference between me terminals of a cell in open circuit is 2.2 volt with resistance of 5 ohm across the terminals of a cell, the terminal potential difference is 1.8 volt. The internal resistance of the cell is:
A)
\[\frac{9}{10}\] ohm
done
clear
B)
\[\frac{10}{9}\] ohm
done
clear
C)
\[\frac{7}{12}\] ohm
done
clear
D)
\[\frac{12}{7}\] ohm
done
clear
View Answer play_arrow
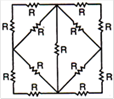
question_answer 39)
Thirteen resistances each of resistance R ohm are connected in the circuit as shown in the figure. The effective resistance between A and B is:
A)
\[\frac{4R}{3}\Omega \]
done
clear
B)
\[2R\Omega \]
done
clear
C)
\[R\Omega \]
done
clear
D)
\[\frac{2R}{3}\Omega \]
done
clear
View Answer play_arrow
question_answer 40)
A group of N cells whose emf varies directly with the internal resistance as per the equation \[{{E}_{N}}=1.5\,{{r}_{N}}\] are connected as shown in the figure above. The current I in the circuit is:
A)
5.1 A
done
clear
B)
0.51 A
done
clear
C)
1.5 A
done
clear
D)
0.15 A
done
clear
View Answer play_arrow
question_answer 41) The temperature coefficient of resistance of a wire is \[0.00125/{{\,}^{o}}C\]. Its resistance is 1 ohm at 300 K. Its resistance will be 2 ohm at:
A)
1127 K
done
clear
B)
1400 K
done
clear
C)
1154 K
done
clear
D)
1100 K
done
clear
View Answer play_arrow
question_answer 42) A potentiometer has uniform potential gradient. The specific resistance of the material of the potentiometer wire is \[{{10}^{-7}}\] ohm-metre and the current passing through it is 0.1 ampere, cross-section of the wire is 10 m. The potential gradient along the potentiometer wire is:
A)
\[{{10}^{-6}}V/m\]
done
clear
B)
\[{{10}^{-4}}V/m\]
done
clear
C)
\[{{10}^{-8}}V/m\]
done
clear
D)
\[{{10}^{-2}}V/m\]
done
clear
View Answer play_arrow
question_answer 43) A fuse wire with radius 1 mm blows at 1.5 ampere. The radius of the fuse wire of the same material to blow at 3A will be:
A)
\[{{3}^{1/4}}mm\]
done
clear
B)
\[{{4}^{1/3}}mm\]
done
clear
C)
\[{{3}^{1/2}}mm\]
done
clear
D)
\[{{2}^{1/3}}mm\]
done
clear
View Answer play_arrow
question_answer 44) A wire in the form of a circular loop of one turn carrying a current produces a magnetic field B at the centre. If the same wire is looped into a coil of two turns and carries the same current, the new value of magnetic induction at the centre is:
A)
3 B
done
clear
B)
5 B
done
clear
C)
4 B
done
clear
D)
2 B
done
clear
View Answer play_arrow
question_answer 45) To send 10% of the main current through a moving coil galvanometer of resistance 99 ohm, the shunt required is:
A)
10 ohm
done
clear
B)
9.9 ohm
done
clear
C)
9 ohm
done
clear
D)
11 ohm
done
clear
View Answer play_arrow
question_answer 46) The magnetic flux linked with a coil at any instant t is given by \[\phi =5{{t}^{3}}-100t+300,\] the emf induced in the coil at \[t=2\] second is:
A)
40 V
done
clear
B)
- 40 V
done
clear
C)
300 V
done
clear
D)
140 V
done
clear
View Answer play_arrow
question_answer 47)
A charged particles moves along the line AB which lies in the same plane of a circular loop of conducting wire as shown in the figure. Then:
A)
no current will be induced in the loop
done
clear
B)
the current induced in the loop will change its direction as the charged particle passes by
done
clear
C)
the current induced will be anticlockwise
done
clear
D)
the current induced, will be clockwise
done
clear
View Answer play_arrow
question_answer 48) The time taken by A.C. of 50 Hz in reaching from zero to the maximum value is:
A)
\[50\times {{10}^{-3}}sec\]
done
clear
B)
\[5\times {{10}^{-3}}sec\]
done
clear
C)
\[1\times {{10}^{-3}}sec\]
done
clear
D)
\[2\times {{10}^{-3}}sec\]
done
clear
View Answer play_arrow
question_answer 49) The ratio of the secondary to the primary turns in a transformer is 3 : 2 and the output power is P. Neglecting all power losses, the input power must be:
A)
P/2
done
clear
B)
P
done
clear
C)
2 P/3
done
clear
D)
3 P/2
done
clear
View Answer play_arrow
question_answer 50) The material used for permanent magnet has:
A)
low retentivity, high coercivity
done
clear
B)
high retentivity, low coercivity
done
clear
C)
high retentivity, high coercivity
done
clear
D)
low retentivity, low coercivity
done
clear
View Answer play_arrow
question_answer 51) A particle of mass M at rest decays into two masses \[{{m}_{1}}\] and \[{{m}_{2}}\] with non zero velocities. The ratio of de-Broglie wavelengths of the particles \[\frac{{{\lambda }_{1}}}{{{\lambda }_{2}}}\] is:
A)
\[\frac{{{m}_{2}}}{{{m}_{1}}}\]
done
clear
B)
\[\frac{{{m}_{1}}}{{{m}_{2}}}\]
done
clear
C)
\[\frac{\sqrt{{{m}_{1}}}}{\sqrt{{{m}_{2}}}}\]
done
clear
D)
\[1:1\]
done
clear
View Answer play_arrow
question_answer 52) For an electron in the second orbit of Bohrs hydrogen atom, the moment of linear momentum is:
A)
\[\pi h\]
done
clear
B)
\[2\pi h\]
done
clear
C)
\[\frac{h}{\pi }\]
done
clear
D)
\[\frac{2h}{\pi }\]
done
clear
View Answer play_arrow
question_answer 53) If elements with principal quantum number \[n>4\]were not allowed in nature, the number of possible elements would have been:
A)
32
done
clear
B)
60
done
clear
C)
64
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 54) In photoelectric effect, the number of electrons ejected per second is:
A)
proportional to the wavelength of light
done
clear
B)
proportional to the intensity of light
done
clear
C)
proportional to the work function of the metal
done
clear
D)
proportional to the frequency of light
done
clear
View Answer play_arrow
question_answer 55) Half life of a radioactive substance is 20 minutes. The time between 20% and 80% decay will be:
A)
40 minutes
done
clear
B)
20 minutes
done
clear
C)
25 minutes
done
clear
D)
30 minutes
done
clear
View Answer play_arrow
question_answer 56) A hypothetical radioactive nucleus decays according to the following series \[_{72}{{A}^{180}}\xrightarrow{\alpha }{{A}_{1}}\xrightarrow{{{\beta }^{-}}}{{A}_{2}}\xrightarrow{\alpha }{{A}_{3}}\xrightarrow{\gamma }{{A}_{4}}\] If the mass number and atomic number of A are respectively 180 and 72. Then the atomic number and mass number of A will respectively be:
A)
69, 171
done
clear
B)
70, 172
done
clear
C)
68, 172
done
clear
D)
69, 172
done
clear
View Answer play_arrow
question_answer 57) Nucleus A is converted into C through the following reactions: \[A\xrightarrow[{}]{{}}B+\alpha \] [\[\alpha \] - alpha particle \[B\xrightarrow[{}]{{}}C+2\beta \] \[\beta \]-electron] then:
A)
A and B are isotopes
done
clear
B)
A and C are isobars
done
clear
C)
A and B are isobars
done
clear
D)
A and C are isotopes
done
clear
View Answer play_arrow
question_answer 58) If \[m,\,{{m}_{n}}\] and \[{{m}_{p}}\] are the masses of \[_{Z}{{X}^{A}}\]nucleus, neutron and proton respectively:
A)
\[m=\left( A-Z \right){{m}_{n}}+Z{{m}_{p}}\]
done
clear
B)
\[m<\left( A-Z \right){{m}_{n}}+Z{{m}_{p}}\]
done
clear
C)
\[m>\left( A-Z \right){{m}_{n}}+Z{{m}_{p}}\]
done
clear
D)
\[m=\left( A-Z \right){{m}_{p}}+Z{{m}_{n}}\]
done
clear
View Answer play_arrow
question_answer 59) The electrical circuit used to get smooth D.C output from a rectifier circuit is called:
A)
filter
done
clear
B)
oscillator
done
clear
C)
logic gates
done
clear
D)
amplifier
done
clear
View Answer play_arrow
question_answer 60) In the case of constants \[\alpha \] and \[\beta \] of a transistor:
A)
\[\alpha =\beta \]
done
clear
B)
\[\beta <1,\alpha >1\]
done
clear
C)
\[\alpha \beta =1\]
done
clear
D)
\[\beta >1,\alpha <1\]
done
clear
View Answer play_arrow
question_answer 61) A colour less crystalline salt \[x\] is soluble in dilute \[HCl\]. On adding \[NaOH\] solution, it gives a white precipitate which is insoluble in excess of \[NaOH\]. \[x\] is :
A)
\[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\]
done
clear
B)
\[ZnS{{O}_{4}}\]
done
clear
C)
\[MgS{{O}_{4}}\]
done
clear
D)
\[SnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 62) Which metal is used to make alloy steel for armour plates, safes and helmets?
A)
Al
done
clear
B)
Mn
done
clear
C)
Cr
done
clear
D)
Pb
done
clear
View Answer play_arrow
question_answer 63) lodoform test is not answered by:
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 64) A gaseous carbon compound is soluble in dilute \[HCl\]. The solution on treating with \[NaN{{O}_{2}}\] gives off nitrogen leaving behind a solution which smells of wood spirit. The carbon compound is:
A)
\[HCHO\]
done
clear
B)
CO
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following statements is incorrect regarding benzyl chloride?
A)
It gives white precipitate with alcoholic \[AgN{{O}_{3}}\]
done
clear
B)
It is an aromatic compound with substitution in the side chain
done
clear
C)
It undergoes nucleophilic substitution reaction
done
clear
D)
It is less reactive than vinyl chloride
done
clear
View Answer play_arrow
question_answer 66) Enthalpy of formation of HF and \[HCl\] are -161 kJ and -92 kJ respectively. Which of the following statements is incorrect?
A)
\[HCl\] is more stable than HF
done
clear
B)
HF and \[HCl\] arc exothermic compounds
done
clear
C)
The affinity of fluorine to hydrogen is greater than the affinity of chlorine to hydrogen
done
clear
D)
HF is more stable than \[HCl\]
done
clear
View Answer play_arrow
question_answer 67) Heat liberated with 100 ml of 1 N \[NaOH\] is neutralised by 300 ml of IN \[HCl\]:
A)
11.56 kJ
done
clear
B)
5.73 kJ
done
clear
C)
22.92 kJ
done
clear
D)
17.19 kJ
done
clear
View Answer play_arrow
question_answer 68) For a reaction \[A+B\xrightarrow{{}}C+D\], if concentration of A is doubled without altering that of B, rate doubles. If concentration of B is increased nine times without altering that of A, rate triples. Order of the reaction is :
A)
2
done
clear
B)
1
done
clear
C)
\[1\frac{1}{2}\]
done
clear
D)
\[1\frac{1}{3}\]
done
clear
View Answer play_arrow
question_answer 69) In Goldschmidt aluminothermic process, thermite contains :
A)
3 parts of \[A{{l}_{2}}{{O}_{3}}\] and 4 parts of \[Al\]
done
clear
B)
3 parts of \[F{{e}_{2}}{{O}_{3}}\] and 2 parts of \[Al\]
done
clear
C)
3 parts of \[F{{e}_{2}}{{O}_{3}}\] and 1 part of \[Al\]
done
clear
D)
1 part of \[F{{e}_{2}}{{O}_{3}}\] and 1 part of \[Al\]
done
clear
View Answer play_arrow
question_answer 70) The structure of orthophosphoric acid is:
A)
\[H-O-\overset{\begin{smallmatrix} O \\ \uparrow \end{smallmatrix}}{\mathop{\underset{\begin{align} & | \\ & O \\ & | \\ & H \\ \end{align}}{\mathop{P}}\,}}\,-O-H\]
done
clear
B)
\[O\leftarrow \overset{\begin{smallmatrix} O \\ | \end{smallmatrix}}{\mathop{\underset{\begin{align} & | \\ & O \\ & | \\ & H \\ \end{align}}{\mathop{P}}\,}}\,-O-H\]
done
clear
C)
\[O\leftarrow \overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{P}}\,}}\,-O-H\]
done
clear
D)
\[H-OP=O\]
done
clear
View Answer play_arrow
question_answer 71) A galvanic cell is constructed using the redox reaction, \[\frac{1}{2}{{H}_{2}}(g)+AgCl(s){{H}^{+}}(aq)+C{{l}^{-}}\]\[(aq)+Ag(s)\]it is represented as :
A)
\[Pt|{{H}_{2}}(g)|HCl(sol.n)|l\,AgN{{O}_{3}}(sol.n)|Ag\]
done
clear
B)
\[Ag|AgCl(s)|KCl(sol.n)|l\,HCl(sol.n)\]\[,\,{{H}_{2}}(g)|Pt\]
done
clear
C)
\[Pt|{{H}_{2}}(g)|KCl(sol.n)|l\,AgCl(s)\,l\,Ag\]
done
clear
D)
\[Pt|{{H}_{2}}(g)|HCl(sol.n)|l\,AgCl(s)\,l\,Ag\]
done
clear
View Answer play_arrow
question_answer 72) Same amount of electric current is passed through solutions of \[AgN{{O}_{3}}\] and \[HCl\]. If 1.08 g of silver is obtained in the first case, the amount of hydrogen liberated at S.T.P. in the second case is :
A)
\[224\,c{{m}^{3}}\]
done
clear
B)
1.008 g
done
clear
C)
\[112\,c{{m}^{3}}\]
done
clear
D)
\[22400\,c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 73) The flame colours of metal ions are due to:
A)
Frenkel defect
done
clear
B)
Schottky defect
done
clear
C)
Metal deficiency defect
done
clear
D)
Metal excess defect
done
clear
View Answer play_arrow
question_answer 74) The order of reactivities of methyl halides in the formation of Grignard reagent is :
A)
\[C{{H}_{3}}>C{{H}_{3}}Br>C{{H}_{3}}Cl\]
done
clear
B)
\[C{{H}_{3}}Cl>C{{H}_{3}}Br>C{{H}_{3}}I\]
done
clear
C)
\[C{{H}_{3}}Br>C{{H}_{3}}CI>C{{H}_{3}}I\]
done
clear
D)
\[C{{H}_{3}}Br>C{{H}_{3}}I>C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 75) The reaction of an organic compound with ammonia followed by nitration of the product gives a powerful explosive, called RDX. The organic compound is :
A)
phenol
done
clear
B)
toluene
done
clear
C)
glycerine
done
clear
D)
formaldehyde
done
clear
View Answer play_arrow
question_answer 76) A signature, written in carbon pencil weighs 1 mg. What is the number of carbon atoms present in the signature?
A)
\[5.02\times {{10}^{23}}\]
done
clear
B)
\[5.02\times {{10}^{20}}\]
done
clear
C)
\[6.02\times {{10}^{20}}\]
done
clear
D)
\[0.502\times {{10}^{20}}\]
done
clear
View Answer play_arrow
question_answer 77) \[N{{H}_{3}}\] and \[HCl\] gas are introduced simultaneously from the two ends of a long tube. A white ring of \[N{{H}_{4}}Cl\] appears first:
A)
nearer to the \[HCl\] end
done
clear
B)
at the centre of the tube
done
clear
C)
throughout the tube
done
clear
D)
nearer to the \[N{{H}_{3}}\] end
done
clear
View Answer play_arrow
question_answer 78) A gas formed by the action of alcoholic \[KOH\]on ethyl iodide, decolourises alkaline \[KMn{{O}_{4}}\]. The gas is :
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following is not a characteristic of chemisorption?
A)
\[\Delta H\]is of the order of 400 kJ
done
clear
B)
Adsorption is irreversible
done
clear
C)
Adsorption may be multimolecular layer
done
clear
D)
Adsorption is specific
done
clear
View Answer play_arrow
question_answer 80) The concentration of electrolyte required to coagulate a given amount of \[A{{s}_{2}}{{S}_{3}}\] sol. is minimum in the case of :
A)
magnesium nitrate
done
clear
B)
potassium nitrate
done
clear
C)
potassium sulphate
done
clear
D)
aluminium nitrate
done
clear
View Answer play_arrow
question_answer 81) Identify the organic compound which, on heating with strong solution of \[NaOH\], partly converted into an acid salt and partly into alcohol :
A)
Benzyl alcohol
done
clear
B)
Acetaldehyde
done
clear
C)
Acetone
done
clear
D)
Benzaldehyde
done
clear
View Answer play_arrow
question_answer 82) The process by which synthesis of protein takes place based on the genetic information present in w-RNA is called :
A)
translation
done
clear
B)
transcription
done
clear
C)
replication
done
clear
D)
messenger hypothesis
done
clear
View Answer play_arrow
question_answer 83) The enthalpies of formation of \[A{{l}_{2}}{{O}_{3}}\] and \[C{{r}_{2}}{{O}_{3}}\] are \[-1596\text{ }kJ\] and \[-1134kJ\] respectively. \[\Delta H\] for the reaction, \[2Al+C{{r}_{2}}{{O}_{3}}\xrightarrow{{}}2Cr+A{{l}_{2}}{{O}_{3}}\] is:
A)
-2730 kJ
done
clear
B)
-462 kJ
done
clear
C)
-1365 kJ
done
clear
D)
+2730 kJ
done
clear
View Answer play_arrow
question_answer 84) The gaseous reaction \[A+B2C+D+Q\] is most favoured at :
A)
low temperature and high pressure
done
clear
B)
high temperature and high pressure
done
clear
C)
high temperature and low pressure
done
clear
D)
low temperature and low pressure
done
clear
View Answer play_arrow
question_answer 85) Temperature coefficient of a reaction is 2. When temperature is increased from \[{{30}^{o}}C\] to\[{{100}^{o}}C\], rate of the reaction increases by :
A)
128 times
done
clear
B)
100 times
done
clear
C)
500 times
done
clear
D)
250 times
done
clear
View Answer play_arrow
question_answer 86) The volume of water to be added to \[\frac{N}{2}HCl\]to prepare \[500\,c{{m}^{3}}\] of \[\frac{N}{10}\] solution is :
A)
\[450\text{ }c{{m}^{3}}\]
done
clear
B)
\[100\text{ }c{{m}^{3}}\]
done
clear
C)
\[45\text{ }c{{m}^{3}}\]
done
clear
D)
\[400\text{ }c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 87) The equivalent weight of a certain trivalent element is 20. Molecular, weight of its oxide is:
A)
152
done
clear
B)
56
done
clear
C)
168
done
clear
D)
68
done
clear
View Answer play_arrow
question_answer 88) Identify the reaction that doesnt take place during the smelting process of copper extraction :
A)
\[2FeS+3{{O}_{2}}\xrightarrow{{}}2FeO+3S{{O}_{2}}\uparrow \]
done
clear
B)
\[C{{u}_{2}}O+FeS\xrightarrow{{}}C{{u}_{2}}S+FeO\]
done
clear
C)
\[2C{{u}_{2}}S+3{{O}_{2}}\xrightarrow{{}}2C{{u}_{2}}O+2S{{O}_{2}}\uparrow \]
done
clear
D)
\[FeO+Si{{O}_{2}}\xrightarrow{{}}FeSi{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 89) Pick out the complex compound in which the central metal atom obeys EAN rule strictly :
A)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
B)
\[{{K}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[[Cr{{({{H}_{2}}O)}_{6}}]C{{l}_{3}}\]
done
clear
D)
\[[Cu{{(N{{H}_{3}})}_{4}}]S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 90) In a reversible reaction, the catalyst :
A)
increases the activation energy of the backward reaction
done
clear
B)
increases the activation energy of the forward reaction
done
clear
C)
decreases the activation energy of both, forward and backward reaction
done
clear
D)
decreases the activation energy of forward reaction
done
clear
View Answer play_arrow
question_answer 91) Solubility product of a salt AB is \[1\times {{10}^{-8}}\] in a solution in which concentration of A is \[{{10}^{-3}}M\]. The salt will precipitate when the concentration of B becomes more than :
A)
\[{{10}^{-4}}M\]
done
clear
B)
\[{{10}^{-7}}M\]
done
clear
C)
\[{{10}^{-6}}M\]
done
clear
D)
\[{{10}^{-5}}M\]
done
clear
View Answer play_arrow
question_answer 92) The standard reduction potentials of Zn and Ag in water at 298 K are, \[Z{{n}^{+2}}+2{{e}^{-}}Zn;\] \[{{E}^{o}}=-0.76\,V\] and \[A{{g}^{+}}+{{e}^{-}}Ag;\]\[{{E}^{o}}=+0.80\,V\]. Which of the following reactions take place?
A)
\[Z{{n}^{+2}}(aq)+2Ag(s)\xrightarrow{{}}2A{{g}^{+}}(aq)+Zn(s)\]
done
clear
B)
\[Zn(s)+2A{{g}^{+}}(aq)\xrightarrow{{}}Z{{n}^{+2}}(aq)+2Ag(s)\]
done
clear
C)
\[Z{{n}^{+2}}(aq)+A{{g}^{+}}(aq)\xrightarrow{{}}Zn(s)+Ag(s)\]
done
clear
D)
\[Zn(s)+Ag(s)\xrightarrow{{}}Z{{n}^{+2}}(aq)+A{{g}^{+}}(aq)\]
done
clear
View Answer play_arrow
question_answer 93) The ratio of cationic radius to anionic radius in an ionic crystal is greater than 0.732. Its co-ordination number is :
A)
6
done
clear
B)
8
done
clear
C)
1
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 94) Dacron is obtained by the condensation polymerization of :
A)
Dimethyl terephthalate and ethylene glycol
done
clear
B)
Terephthalic acid and formaldehyde
done
clear
C)
Phenol and phthalic acid
done
clear
D)
Phenol and formaldehyde
done
clear
View Answer play_arrow
question_answer 95) 4-chloro-3, 5-dimethyl phenol is called :
A)
Chloramphenicol
done
clear
B)
Paracetamol
done
clear
C)
Barbital
done
clear
D)
Dettol
done
clear
View Answer play_arrow
question_answer 96) The percentage s-character of the hybrid orbitals in methane, ethene and ethyne are respectively :
A)
25, 33, 50
done
clear
B)
25, 50, 75
done
clear
C)
50, 75, 100
done
clear
D)
10, 20, 40
done
clear
View Answer play_arrow
question_answer 97) In the manufacture of sulphuric acid by contact process, Tyndall box is used to :
A)
filter dust particles
done
clear
B)
remove impurities
done
clear
C)
convert \[S{{O}_{2}}\] to \[S{{O}_{3}}\]
done
clear
D)
test the presence of dust particles
done
clear
View Answer play_arrow
question_answer 98) The pH value of gastric juice in human stomach is about 1.8 and in the small intestine it is about 7.8. The \[p{{K}_{a}}\] value of aspirin is 3.5. Aspirin will be:
A)
completely ionized in the small intestine and in the stomach
done
clear
B)
unionised in the small intestine and in the stomach
done
clear
C)
ionised in the small intestine and almost unionised in the stomach
done
clear
D)
ionised in the stomach and almost unionised in the small, intestine
done
clear
View Answer play_arrow
question_answer 99) The number of a and P particles emitted during the transformation of \[_{90}T{{h}^{232}}\] to \[_{82}P{{b}^{208}}\] are respectively :
A)
4, 2
done
clear
B)
2, 2
done
clear
C)
8, 6
done
clear
D)
6, 4
done
clear
View Answer play_arrow
question_answer 100) When chlorine is passed through warm benzene in presence of the sunlight, the product obtained is :
A)
Benzotrichloride
done
clear
B)
Chlorobenzene
done
clear
C)
Gammexane
done
clear
D)
D.D.T.
done
clear
View Answer play_arrow
question_answer 101) Ethyl benzoate reacts with \[PC{{l}_{5}}\] to give :
A)
\[{{C}_{2}}{{H}_{5}}Cl+{{C}_{6}}{{H}_{5}}COCl+POC{{l}_{3}}+HCl\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}Cl+{{C}_{6}}{{H}_{5}}COCl+POC{{l}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COCl+{{C}_{6}}{{H}_{5}}COCl+POC{{l}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}Cl+{{C}_{6}}{{H}_{5}}COOH+POC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 102) Pick out the statement which is not relevant in the discussion of colloids :
A)
Sodium aluminium silicate is used in the softening of hard water
done
clear
B)
Potash alum is used in shaving rounds and as a styptic in medicine
done
clear
C)
Artificial rain is caused by throwing electrified sand on the clouds from an aeroplane
done
clear
D)
Deltas are formed at a place where the river pours its water into the sea
done
clear
View Answer play_arrow
question_answer 103) A wooden box excavated from Indus valley had an activity of \[1.18\times {{10}^{13}}\] disintegration per minute per gm. of carbon. What is the approximate age of this civilisation?
A)
4000 years
done
clear
B)
5700 years
done
clear
C)
8100 years
done
clear
D)
6000 years
done
clear
View Answer play_arrow
question_answer 104) For a reaction if \[{{K}_{p}}>{{K}_{c}}\], the forward reaction is favoured by :
A)
low pressure
done
clear
B)
high pressure
done
clear
C)
high temperature
done
clear
D)
low temperature
done
clear
View Answer play_arrow
question_answer 105) In a lime kiln, to get higher yield of \[C{{O}_{2}}\], the measure that can be taken is :
A)
to remove CaO
done
clear
B)
to add more \[CaC{{O}_{3}}\]
done
clear
C)
to maintain high temperature
done
clear
D)
to pump out \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 106) What is the volume of 20 volume \[{{H}_{2}}{{O}_{2}}\] required to get \[5000\text{ }c{{m}^{3}}\] of oxygen at S.T.R?
A)
\[250\text{ }c{{m}^{3}}\]
done
clear
B)
\[50\text{ }c{{m}^{3}}\]
done
clear
C)
\[100\text{ }c{{m}^{3}}\]
done
clear
D)
\[125\text{ }c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 107) The IUPAC name of \[{{(C{{H}_{3}})}_{3}}C-CH=C{{H}_{2}}\] is:
A)
1, 1, l-trimethyl-2-propene
done
clear
B)
3, 3, 3-trimethyl-2-propene
done
clear
C)
2, 2-dimethyl-3-butene
done
clear
D)
3, 3-dimethyl-l-butene
done
clear
View Answer play_arrow
question_answer 108) Railway wagon axles are made by heating iron rods embedded in charcoal powder. This process is known as:
A)
Tempering
done
clear
B)
Case hardening
done
clear
C)
Sherardising
done
clear
D)
Annealing
done
clear
View Answer play_arrow
question_answer 109) Thomas slag is ...
A)
\[CaSi{{O}_{3}}\]
done
clear
B)
\[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\]
done
clear
C)
\[MnSi{{O}_{3}}\]
done
clear
D)
\[CaC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 110) Urea is preferred to ammonium sulphate as a nitrogenous fertilizer because :
A)
it is more soluble in water
done
clear
B)
it is cheaper than ammonium sulphate
done
clear
C)
it is quite stable
done
clear
D)
it does not cause acidity in the soil
done
clear
View Answer play_arrow
question_answer 111) Two gas cylinders having same capacity have been filled with 44 g of \[{{H}_{2}}\] and 44 g of \[C{{O}_{2}}\]respectively. If the pressure in \[C{{O}_{2}}\] cylinder is 1 atmosphere at a particular temperature, the pressure in the hydrogen cylinder at the same temperature is :
A)
2 atmosphere
done
clear
B)
1 atmosphere
done
clear
C)
22 atmosphere
done
clear
D)
44 atmosphere
done
clear
View Answer play_arrow
question_answer 112) Angular momentum of an electron in the nth orbit of hydrogen atom is given by :
A)
\[\frac{nh}{2\pi }\]
done
clear
B)
\[nh\]
done
clear
C)
\[\frac{2\pi }{nh}\]
done
clear
D)
\[\frac{\pi }{2nh}\]
done
clear
View Answer play_arrow
question_answer 113) The element with atomic number 36 belongs to ......... block in the periodic table :
A)
\[p\]
done
clear
B)
\[s\]
done
clear
C)
\[f\]
done
clear
D)
\[d\]
done
clear
View Answer play_arrow
question_answer 114) The function of \[AlC{{l}_{3}}\] in Friedel-Crafts reaction is :
A)
to absorb \[HCl\]
done
clear
B)
to absorb water
done
clear
C)
to produce nucleophiie
done
clear
D)
to produce electrophile
done
clear
View Answer play_arrow
question_answer 115) An important reaction of acetone is auto condensation in presence of concentrated sulphuric acid to give the aromatic compound ......
A)
mesitylene
done
clear
B)
mesityl oxide
done
clear
C)
trioxan
done
clear
D)
phorone
done
clear
View Answer play_arrow
question_answer 116) Kinetic energy of one mole of an ideal gas at 300 K in kJ is :
A)
3.74
done
clear
B)
348
done
clear
C)
34.8
done
clear
D)
3.48
done
clear
View Answer play_arrow
question_answer 117) The tripeptide hormone present in most living cells is :
A)
glutathione
done
clear
B)
glutamine
done
clear
C)
oxytocin
done
clear
D)
ptyalin
done
clear
View Answer play_arrow
question_answer 118) Phenol\[\xrightarrow{NaN{{O}_{2}}|{{H}_{2}}S{{O}_{4}}}B\xrightarrow{{{H}_{2}}O}C\xrightarrow{NaOH}D\]name of the reaction is :
A)
Liebermanns reaction
done
clear
B)
Phthalein fusion test
done
clear
C)
Reimer Tiemann reaction
done
clear
D)
Schotten-Baumann reaction
done
clear
View Answer play_arrow
question_answer 119) Energy is stored in our body in the form of:
A)
ATP
done
clear
B)
ADP
done
clear
C)
Fats
done
clear
D)
Carbohydrates
done
clear
View Answer play_arrow
question_answer 120) An organic compound answers Molischs test as well as Benedicts test. But it does not answer Scliwanoffs test. Most probably, it is :
A)
sucrose
done
clear
B)
protein
done
clear
C)
fructose
done
clear
D)
maltose
done
clear
View Answer play_arrow
question_answer 121) In Z, the set of all integers, the inverse of -7 w.r.t. *defined by \[a*b=a+b+7\] for all \[a,\,b\,\in Z\] is :
A)
-14
done
clear
B)
7
done
clear
C)
14
done
clear
D)
-7
done
clear
View Answer play_arrow
question_answer 122) \[(p\wedge \,\,\,\,\,\,\,p)\wedge (\sim p\wedge q)\]is:
A)
a tautology
done
clear
B)
a contradiction
done
clear
C)
tautology and contradiction
done
clear
D)
neither a tautology nor a contradiction
done
clear
View Answer play_arrow
question_answer 123) The number of words that can be formed out of the letters of the word ARTICLE so that the vowels occupy even places is :
A)
574
done
clear
B)
36
done
clear
C)
754
done
clear
D)
144
done
clear
View Answer play_arrow
question_answer 124) In \[\Delta \,ABC\] if \[\left| \begin{matrix} 1 & a & b \\ 1 & c & a \\ 1 & b & c \\ \end{matrix} \right|=0\] then\[{{\sin }^{2}}A+{{\sin }^{2}}B+{{\sin }^{2}}C\] is equal to :
A)
\[\frac{4}{9}\]
done
clear
B)
\[\frac{9}{4}\]
done
clear
C)
\[3\sqrt{3}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 125) 7th term of an A.P. is 40. Then the sum of first 13 terms is :
A)
520
done
clear
B)
53
done
clear
C)
2080
done
clear
D)
1040
done
clear
View Answer play_arrow
question_answer 126) The coefficient of \[{{x}^{32}}\]in the expansion of\[{{\left( {{x}^{4}}-\frac{1}{{{x}^{3}}} \right)}^{15}}\]is:
A)
\[^{-15}{{C}_{3}}\]
done
clear
B)
\[^{15}{{C}_{4}}\]
done
clear
C)
\[^{-15}{{C}_{5}}\]
done
clear
D)
\[^{15}{{C}_{2}}\]
done
clear
View Answer play_arrow
question_answer 127) Which of the following is a subgroup of\[a=\text{ }\!\!\{\!\!\text{ }0,1,2,3,4,5\text{ }\!\!\}\!\!\text{ },\] under addition modules 6?
A)
{0, 2}
done
clear
B)
{0, 1}
done
clear
C)
{0, 4}
done
clear
D)
{0, 3}
done
clear
View Answer play_arrow
question_answer 128) Assuming that the sums and products given below are defined which of the following is not true for matrices?
A)
\[AB=AC\] does not imply \[B=C\]
done
clear
B)
\[A+B=B+A\]
done
clear
C)
\[(AB)=BA\]
done
clear
D)
\[AB=0\] implies \[A=0\] or \[B=0\]
done
clear
View Answer play_arrow
question_answer 129) Inverse of the matrix \[\left[ \begin{matrix} 1 & -2 \\ 3 & 4 \\ \end{matrix} \right]\] is :
A)
\[\frac{1}{10}\left[ \begin{matrix} 1 & -2 \\ 3 & 4 \\ \end{matrix} \right]\]
done
clear
B)
\[\frac{1}{10}\left[ \begin{matrix} 4 & 2 \\ -3 & 1 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 4 & 2 \\ -3 & 1 \\ \end{matrix} \right]\]
done
clear
D)
\[\frac{1}{10}\left[ \begin{matrix} 4 & -2 \\ -3 & 1 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 130) If the vectors \[4\hat{i}+11\hat{j}+m\hat{k}\], \[7\hat{i}+2\hat{j}+6\hat{k}\] and \[i+5j+4k\] arc coplanar then m is equal to :
A)
0
done
clear
B)
38
done
clear
C)
-10
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 131) \[\left| \begin{matrix} {{b}^{2}}{{c}^{2}} & bc & b+c \\ {{c}^{2}}{{a}^{2}} & ca & c+a \\ {{a}^{2}}{{b}^{2}} & ab & a+b \\ \end{matrix} \right|\] is equal to :
A)
\[\frac{1}{abc}(ab+bc+ca)\]
done
clear
B)
\[ab+bc+ca\]
done
clear
C)
0
done
clear
D)
\[a+b+c\]
done
clear
View Answer play_arrow
question_answer 132) In \[\Delta \,ABC\] if \[\frac{b+c}{11}=\frac{c+a}{12}=\frac{a+b}{13}\] then \[\cos C\]is equal to :
A)
\[\frac{5}{7}\]
done
clear
B)
\[\frac{7}{5}\]
done
clear
C)
\[\frac{16}{17}\]
done
clear
D)
\[\frac{17}{36}\]
done
clear
View Answer play_arrow
question_answer 133) The value of \[\frac{\tan {{70}^{o}}-\tan {{20}^{o}}}{\tan {{50}^{o}}}\] is equal to:
A)
2
done
clear
B)
1
done
clear
C)
0
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 134) \[\cos {{1}^{o}}+\cos {{2}^{o}}+\cos {{3}^{o}}+....+\cos {{180}^{o}}\] is equal to :
A)
1
done
clear
B)
0
done
clear
C)
2
done
clear
D)
-1
done
clear
View Answer play_arrow
question_answer 135) If \[\left| \vec{a}\times \vec{b} \right|=4\] and \[\left| \vec{a}\,.\,\vec{b} \right|=2\] then \[{{\left| {\vec{a}} \right|}^{2}}{{\left| {\vec{b}} \right|}^{2}}\] is equal to :
A)
6
done
clear
B)
2
done
clear
C)
20
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 136) \[\underset{x\to 0}{\mathop{\lim }}\,\,\,\,{{(1-ax)}^{1/x}}\] is equal to :
A)
\[{{e}^{-a}}\]
done
clear
B)
e
done
clear
C)
\[{{e}^{a}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 137) Which of the following is not a proposition :
A)
3 is prime
done
clear
B)
\[\sqrt{2}\] is irrational
done
clear
C)
Mathematics is interesting
done
clear
D)
5 is an even integer
done
clear
View Answer play_arrow
question_answer 138) \[\underset{n\to \infty }{\mathop{\lim }}\,\,{{({{3}^{n}}+{{4}^{n}})}^{1/n}}\] is equal to :
A)
4
done
clear
B)
3
done
clear
C)
e
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 139) The distance between the directories of the hyperbola \[x=8\sec \theta ,\,y=8\,\tan \theta \] is :
A)
\[8\sqrt{2}\]
done
clear
B)
\[16\sqrt{2}\]
done
clear
C)
\[4\sqrt{2}\]
done
clear
D)
\[6\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 140) The general solution of the equation \[\tan 2\theta \,.\,\tan \theta =1\] for \[n\in z\] is, \[\theta \] is equal to:
A)
\[(2n+1)\frac{\pi }{4}\]
done
clear
B)
\[(2n+1)\frac{\pi }{6}\]
done
clear
C)
\[(2n+1)\frac{\pi }{2}\]
done
clear
D)
\[\frac{1}{1}(2n+1)\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 141) \[\sin \left( \frac{1}{2}{{\cos }^{-1}}\frac{4}{5} \right)\] is equal to :
A)
\[-\frac{1}{\sqrt{10}}\]
done
clear
B)
\[\frac{1}{\sqrt{10}}\]
done
clear
C)
\[-\frac{1}{10}\]
done
clear
D)
\[\frac{1}{10}\]
done
clear
View Answer play_arrow
question_answer 142) The angle between the vectors \[\vec{a}+\vec{b}\] and \[\vec{a}-\vec{b}\] when \[a=(1,1,4)\] and \[b=(1,-1,4)\]is:
A)
\[{{45}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[{{15}^{o}}\]
done
clear
D)
\[{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 143) The angle between the lines in \[{{x}^{2}}-xy-6{{y}^{2}}-7x+31y-18=0\] is :
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{30}^{o}}\]
done
clear
D)
\[{{90}^{o}}\]
done
clear
View Answer play_arrow
question_answer 144) The equation of line bisecting perpendicularly the segment joining the points (-4, 6) and (8,8) is :
A)
\[y=7\]
done
clear
B)
\[6x+y-19=0\]
done
clear
C)
\[x+2y-7=0\]
done
clear
D)
\[6x+2y-19=0\]
done
clear
View Answer play_arrow
question_answer 145) If the distance of any point P from the points \[A\,(a+b,\,\,a-b)\] and B \[B\,(a-b,\,\,a+b)\] are equal then the locus of P is :
A)
\[ax+by=0\]
done
clear
B)
\[x-y=0\]
done
clear
C)
\[x+y=0\]
done
clear
D)
\[bx-ay=0\]
done
clear
View Answer play_arrow
question_answer 146) If p is the length of the perpendicular from the origin on the line whose intercepts on the axes are a and b then :
A)
\[{{p}^{2}}={{a}^{2}}+{{b}^{2}}\]
done
clear
B)
\[{{p}^{2}}={{a}^{2}}-{{b}^{2}}\]
done
clear
C)
\[\frac{1}{{{p}^{2}}}=\frac{1}{{{a}^{2}}}+\frac{1}{{{b}^{2}}}\]
done
clear
D)
\[\frac{1}{{{p}^{2}}}=\frac{1}{{{a}^{2}}}-\frac{1}{{{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 147) If the slope of one of the lines given by \[a{{x}^{2}}+2hxy+b{{y}^{2}}=0\] is 5 times the other then:
A)
\[5{{h}^{2}}=9ab\]
done
clear
B)
\[5{{h}^{2}}=ab\]
done
clear
C)
\[{{h}^{2}}=ab\]
done
clear
D)
\[9{{h}^{2}}=5ab\]
done
clear
View Answer play_arrow
question_answer 148) \[\int_{0}^{2\pi }{(\sin x+|\sin x|dx}\] is equal to :
A)
4
done
clear
B)
0
done
clear
C)
1
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 149) \[\int_{0}^{\pi }{\frac{x\,dx}{{{a}^{2}}{{\cos }^{2}}x+{{b}^{2}}{{\sin }^{2}}x}}\] is equal to :
A)
\[\frac{\pi }{2ab}\]
done
clear
B)
\[\frac{\pi }{ab}\]
done
clear
C)
\[\frac{{{\pi }^{2}}}{2ab}\]
done
clear
D)
\[\frac{{{\pi }^{2}}}{ab}\]
done
clear
View Answer play_arrow
question_answer 150) The differential equation for which \[{{\sin }^{-1}}x+{{\sin }^{-1}}y=c\] is given by :
A)
\[\sqrt{1-{{x}^{2}}}dy+\sqrt{1-{{y}^{2}}}\,dx=0\]
done
clear
B)
\[\sqrt{1-{{x}^{2}}}dx+\sqrt{1-{{y}^{2}}}\,dy=0\]
done
clear
C)
\[\sqrt{1-{{x}^{2}}}dx-\sqrt{1-{{y}^{2}}}\,dy=0\]
done
clear
D)
\[\sqrt{1-{{x}^{2}}}dy-\sqrt{1-{{y}^{2}}}\,dx=0\]
done
clear
View Answer play_arrow
question_answer 151) The derivative of \[{{\cos }^{-1}}\left( \frac{1-{{x}^{2}}}{1+{{x}^{2}}} \right)\] w.r.t. \[{{\cos }^{-1}}\left( \frac{1-{{x}^{2}}}{1+{{x}^{2}}} \right)\]is :
A)
\[\frac{3}{2}\]
done
clear
B)
1
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[\frac{2}{3}\]
done
clear
View Answer play_arrow
question_answer 152) If \[x=a\,(\theta -\sin \theta ),\,y=a\,(1-\cos \theta )\] then \[\frac{dy}{dx}\] is equal to :
A)
\[\cot \theta /2\]
done
clear
B)
\[\tan \theta /2\]
done
clear
C)
\[\frac{1}{2}\cos e{{c}^{2}}\frac{\theta }{2}\]
done
clear
D)
\[-\frac{1}{2}\cos e{{c}^{2}}\frac{\theta }{2}\]
done
clear
View Answer play_arrow
question_answer 153) If \[y=1-x+\frac{{{x}^{2}}}{2!}-\frac{{{x}^{3}}}{3!}+\frac{{{x}^{4}}}{4\,!}....\]then \[\frac{{{d}^{2}}y}{d{{x}^{2}}}\]is equal to :
A)
\[-x\]
done
clear
B)
\[x\]
done
clear
C)
\[y\]
done
clear
D)
\[-y\]
done
clear
View Answer play_arrow
question_answer 154) If \[\sqrt{x}+\frac{1}{\sqrt{x}}=2\,\cos \theta \] then \[{{x}^{6}}+{{x}^{-6}}\] is equal to:
A)
\[2\cos 12\theta \]
done
clear
B)
\[2\cos 6\,\theta \]
done
clear
C)
\[2\sin 3\,\theta \]
done
clear
D)
\[2\cos 3\,\theta \]
done
clear
View Answer play_arrow
question_answer 155) The slope of the tangent to the curve\[x=3{{t}^{2}}+1,\,y={{t}^{3}}-1\] at \[x=1\] is :
A)
\[\frac{1}{2}\]
done
clear
B)
0
done
clear
C)
-2
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 156) Which of the following is a fourth root \[\frac{1}{2}+\frac{\sqrt{3}}{2}\]?
A)
\[cis\frac{\pi }{12}\]
done
clear
B)
\[cis\frac{\pi }{2}\]
done
clear
C)
\[cis\frac{\pi }{6}\]
done
clear
D)
\[cis\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 157) If \[f(a)=2f(a)=1,\,g(a)=3,\,g(a)=-1\] then \[\underset{x\to a}{\mathop{\lim }}\,\frac{f(a)\,g\,(x)-f(x)\,g\,(a)}{x-a}\] equal to :
A)
6
done
clear
B)
1
done
clear
C)
-1
done
clear
D)
-5
done
clear
View Answer play_arrow
question_answer 158) The rate of change of the surface area of the sphere of radius r when the radius is increasing at the rate of 2 cm/sec is proportional to :
A)
\[\frac{1}{{{r}^{2}}}\]
done
clear
B)
\[\frac{1}{r}\]
done
clear
C)
\[{{r}^{2}}\]
done
clear
D)
\[r\]
done
clear
View Answer play_arrow
question_answer 159) The amplitude of \[\sin \frac{\pi }{5}+i\left( 1-\cos \frac{\pi }{5} \right)\] is :
A)
\[\frac{2\pi }{5}\]
done
clear
B)
\[\frac{\pi }{5}\]
done
clear
C)
\[\frac{\pi }{15}\]
done
clear
D)
\[\frac{\pi }{10}\]
done
clear
View Answer play_arrow
question_answer 160) For the curve \[xy={{c}^{2}}\] the subnormal at any point varies as :
A)
\[{{x}^{3}}\]
done
clear
B)
\[{{x}^{2}}\]
done
clear
C)
\[{{y}^{3}}\]
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 161) The function \[f(x)=\left| x \right|+\frac{\left| x \right|}{x}\] is :
A)
discontinuous at origin because \[\left| x \right|\] is discontinuous there
done
clear
B)
continuous at origin
done
clear
C)
discontinuous at origin because both \[\left| x \right|\] and \[\frac{\left| x \right|}{x}\] are discontinuous there
done
clear
D)
discontinuous at the origin because is discontinuous there.
done
clear
View Answer play_arrow
question_answer 162) \[\int{{{e}^{x}}\left( \frac{1+\sin x}{1+\cos x} \right)dx}\] is equal to :
A)
\[{{e}^{x}}{{\sec }^{2}}\frac{x}{2}+C\]
done
clear
B)
\[{{e}^{x}}\tan \frac{x}{2}+C\]
done
clear
C)
\[{{e}^{x}}\sec \frac{x}{2}+C\]
done
clear
D)
\[{{e}^{x}}+\tan x+C\]
done
clear
View Answer play_arrow
question_answer 163) \[\int{\sqrt{1+\sin \left( \frac{x}{4} \right)}\,dx}\] is equal to :
A)
\[8\,\left( \sin \frac{x}{8}+\cos \frac{x}{8} \right)+C\]
done
clear
B)
\[8\,\left( \sin \frac{x}{8}-\cos \frac{x}{8} \right)+C\]
done
clear
C)
\[8\,\left( \cos \frac{x}{8}-\sin \frac{x}{8} \right)+C\]
done
clear
D)
\[8\,\left( \sin \frac{x}{8}-\cos \frac{x}{8} \right)+C\]
done
clear
View Answer play_arrow
question_answer 164) \[\int_{0}^{\infty }{\frac{x\,dx}{(1+x)\,(1+{{x}^{2}})}}\] is equal to :
A)
\[\frac{\pi }{2}\]
done
clear
B)
0
done
clear
C)
1
done
clear
D)
\[\frac{\pi }{4}\]
done
clear
View Answer play_arrow
question_answer 165) If \[{{I}_{n}}\int{{{(\log \,x)}^{n}}dx}\] then \[{{I}_{n}}+n\,{{I}_{n-1}}\] is equal to :
A)
\[{{(x\,\log \,x)}^{n}}\]
done
clear
B)
\[x\,{{(\log \,x)}^{n}}\]
done
clear
C)
\[n\,{{(\log \,x)}^{n}}\]
done
clear
D)
\[{{(\log \,x)}^{n-1}}\]
done
clear
View Answer play_arrow
question_answer 166) The area included between the parabolas\[x=4y\] and \[y=4x\] is (in square units) :
A)
\[\frac{4}{3}\]
done
clear
B)
\[\frac{1}{3}\]
done
clear
C)
\[\frac{16}{3}\]
done
clear
D)
\[\frac{8}{3}\]
done
clear
View Answer play_arrow
question_answer 167) If \[{{\cos }^{-1}}x+{{\cos }^{-1}}y+{{\cos }^{-1}}z=3\pi \] then\[xy+yz+zx\] is equal to :
A)
1
done
clear
B)
0
done
clear
C)
-3
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 168) Which of the following is a group?
A)
{1, 2, 4, 8} under multiplication
done
clear
B)
\[\{0,\pm \,2,\pm \,4,\pm \,6\text{ }...\}\] under addition
done
clear
C)
\[\{1,-1\}\] under addition
done
clear
D)
{0,1,2,3, 4} under multiplication module 5
done
clear
View Answer play_arrow
question_answer 169) If the circles \[{{x}^{2}}+{{y}^{2}}+2gx+2fy=0\] and\[{{x}^{2}}+{{y}^{2}}+2gx+2fy=0\] touch each other then :
A)
\[ff=gg\]
done
clear
B)
\[fg=fg\]
done
clear
C)
\[{{(fg)}^{2}}={{(fg)}^{2}}\]
done
clear
D)
\[fg=fg\]
done
clear
View Answer play_arrow
question_answer 170) The line \[3x-2y=k\] meets the circle\[{{x}^{2}}+{{y}^{2}}=4{{r}^{2}}\] at only one point if k is equal to:
A)
\[52{{r}^{2}}\]
done
clear
B)
\[20{{r}^{2}}\]
done
clear
C)
\[\frac{20}{9}{{r}^{2}}\]
done
clear
D)
\[\frac{52}{9}{{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 171) In three element group \[\{e,a,b\}\] where e is the identity a b is equal to :
A)
a
done
clear
B)
e
done
clear
C)
ab
done
clear
D)
b
done
clear
View Answer play_arrow
question_answer 172) The locus of a point which moves such that the difference of its distances from two fixed points is always a constant is :
A)
a circle
done
clear
B)
a straight line
done
clear
C)
a hyperbola
done
clear
D)
an ellipse
done
clear
View Answer play_arrow
question_answer 173) The directrix of the parabola\[{{x}^{2}}-4x-8y+12=0\] is :
A)
\[y=0\]
done
clear
B)
\[x=1\]
done
clear
C)
\[y=-1\]
done
clear
D)
\[x=-1\]
done
clear
View Answer play_arrow
question_answer 174) Which of the following is a point on the common chord of the circle\[{{x}^{2}}+{{y}^{2}}+2x-3y+6=0\] and\[{{x}^{2}}+{{y}^{2}}+x-8y-13=0\]
A)
(1, 4)
done
clear
B)
(1, -2)
done
clear
C)
(1, -4)
done
clear
D)
(1, 2)
done
clear
View Answer play_arrow
question_answer 175) The locus of the point of intersection of the perpendicular tangents to ellipse \[\frac{{{x}^{2}}}{9}+\frac{{{y}^{2}}}{4}=1\] is:
A)
\[{{x}^{2}}+{{y}^{2}}=4\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}=9\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}=5\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}=13\]
done
clear
View Answer play_arrow
question_answer 176) The sum of the coefficients in the expansion of \[{{(1+x-3{{x}^{2}})}^{3148}}\] is :
A)
8
done
clear
B)
7
done
clear
C)
1
done
clear
D)
-1
done
clear
View Answer play_arrow
question_answer 177) The relation \[R=((1,1),\text{(}2,\,2\text{)},\,\text{(}3,\,\,3\text{)}\}\] on the set \[\{1,2,3\}\] is :
A)
symmetric only
done
clear
B)
reflexive only
done
clear
C)
an equivalence relation
done
clear
D)
transitive only
done
clear
View Answer play_arrow
question_answer 178) The least remainder when \[{{17}^{30}}\] is divided by 5 is:
A)
2
done
clear
B)
1
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 179) The limiting points of the co-axial system of circles \[{{x}^{2}}+{{y}^{2}}+2\lambda y+4=0\] are :
A)
\[(0\pm 4)\]
done
clear
B)
\[(\pm 2,\,0)\]
done
clear
C)
\[(0,\pm 1)\]
done
clear
D)
\[(0,\pm 2)\]
done
clear
View Answer play_arrow
question_answer 180) The maximum of \[4{{\sin }^{2}}x+3{{\cos }^{2}}x\] is :
A)
4
done
clear
B)
3
done
clear
C)
7
done
clear
D)
5
done
clear
View Answer play_arrow
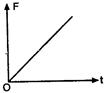
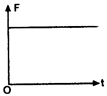
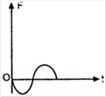
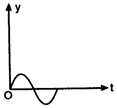 The corresponding force-time graph of the particle is:
The corresponding force-time graph of the particle is:
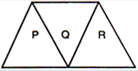 shape and material are now added to P, as shown in the figure. The ray will suffer:
shape and material are now added to P, as shown in the figure. The ray will suffer: