A) III
B) I
C) II
D) All three nitrogen atoms are equally strong nudeophilic centres
Correct Answer: B
Solution :
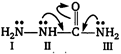
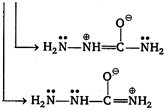 Electron present on nitrogen atom II and III takes part in conjugation with carbonyl group. Hence, due to this reason availability of electron present on these nitrogen atom decreases simultaneously nucleophilicity decreases. While electron present on nitrogen atom (I) do not take part in conjugation and are most nucleophilic in nature.
Electron present on nitrogen atom II and III takes part in conjugation with carbonyl group. Hence, due to this reason availability of electron present on these nitrogen atom decreases simultaneously nucleophilicity decreases. While electron present on nitrogen atom (I) do not take part in conjugation and are most nucleophilic in nature.
You need to login to perform this action.
You will be redirected in
3 sec
