question_answer 1) If two soap bubbles of different radii are connected by a tube, then
A)
air flows from the bigger bubble to the smaller bubble till the sizes became equal
done
clear
B)
air flows from bigger bubble to the smaller bubble till the sizes are interchanged
done
clear
C)
air flows from the smaller bubble to the bigger
done
clear
D)
there is no flow of air
done
clear
View Answer play_arrow
question_answer 2) Which of the following statements is correct for any thermodynamic system?
A)
The internal energy changes in all processes
done
clear
B)
Internal energy and entropy are state functions
done
clear
C)
The change in entropy can never be zero
done
clear
D)
The work done in an adiabatic process is always zero
done
clear
View Answer play_arrow
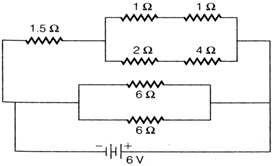
question_answer 3)
The total current supplied to the circuit by the battery is
A)
\[6A\]
done
clear
B)
\[1.33\times {{10}^{-7}}kg\]
done
clear
C)
\[19.8\times {{10}^{-7}}kg\]
done
clear
D)
\[9.9\times {{10}^{-7}}kg\]
done
clear
View Answer play_arrow
question_answer 4) The electrochemical equivalent of metal is \[6.6\times {{10}^{-7}}kg\] per coulomb. The mass of the metal liberated to the cathode when a 3 A current is passed for 2 s, will be
A)
\[1.1\times {{10}^{-7}}kg\]
done
clear
B)
\[{{10}^{o}}C\]
done
clear
C)
\[{{40}^{o}}C\]
done
clear
D)
\[50s\]
done
clear
View Answer play_arrow
question_answer 5) Time taken by a 836 kW heater to heat on litre of water from \[100s\] to \[150s\] is
A)
\[200s\]
done
clear
B)
\[4L\]
done
clear
C)
\[2L\]
done
clear
D)
\[\frac{L}{2}\]
done
clear
View Answer play_arrow
question_answer 6) In an L-C-R circuit, capacitance is changed from C to 2 C. For the resonant frequency to remain unchanged, the inductance should be changed from L to
A)
\[\frac{L}{4}\]
done
clear
B)
\[\propto \text{x}\]
done
clear
C)
\[\propto {{\text{x}}^{2}}\]
done
clear
D)
\[\propto {{\text{x}}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 7) When n-pn transistor is used as an amplifier
A)
electrons move from base to collector
done
clear
B)
holes move from emitter to base
done
clear
C)
electrons move from collector to base
done
clear
D)
holes move from base to emitter
done
clear
View Answer play_arrow
question_answer 8) The maximum number of possible interference maxima from slit-separation equal to twice the wavelength in Youngs double-slit experiment is
A)
infinite
done
clear
B)
five
done
clear
C)
three
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 9) A solid sphere is rotating in free space. If the radius of the sphere is increased, keeping mass same, which one of the following will not be affected?
A)
Moment of inertia
done
clear
B)
Angular momentum
done
clear
C)
Angular velocity
done
clear
D)
Rotational kinetic energy
done
clear
View Answer play_arrow
question_answer 10) The total energy of a particle; executing simple harmonic motion is
A)
\[p+\frac{1}{2}\rho {{\upsilon }^{2}}+\rho gh=k\]
done
clear
B)
\[\frac{4{{v}^{2}}}{5g}\]
done
clear
C)
independent of x
done
clear
D)
\[\frac{4g}{5{{v}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 11) Consider the following equation of Bernoullis theorem \[\frac{{{v}^{2}}}{g}\] (constant) The dimension of k /p are same or that of which of the following?
A)
Thrust
done
clear
B)
Pressure
done
clear
C)
Angle
done
clear
D)
Viscosity
done
clear
View Answer play_arrow
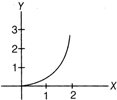
question_answer 12)
If the figure below represent a parabola, identify the physical quantities representing y and X for constant acceleration
A)
X = times, Y = velocity
done
clear
B)
X = velocity, V = time
done
clear
C)
X = time, Y = displacement
done
clear
D)
X = time, Y = acceleration
done
clear
View Answer play_arrow
question_answer 13) A particle is projected with a velocity v such that its range on the horizontal plane is twice the greater height attained by it. The range of the projectile is (where g is acceleration due to gravity)
A)
\[\frac{4{{v}^{2}}}{\sqrt{5}g}\]
done
clear
B)
\[(g=10m/{{s}^{2}})\]
done
clear
C)
\[800N\]
done
clear
D)
\[1200N\]
done
clear
View Answer play_arrow
question_answer 14) A man weighs 80 kg the stands on a weighing scale in a lift which is moving upwards with a uniform acceleration of 5 m/s. What would be the reading on the scale? \[400N\]
A)
\[mg\,sin\,\theta \]
done
clear
B)
\[\frac{{{p}^{2}}{{t}^{2}}}{m}\]
done
clear
C)
zero
done
clear
D)
\[\frac{{{p}^{2}}{{t}^{2}}}{2m}\]
done
clear
View Answer play_arrow
question_answer 15) An inclined plane has an inclination \[\theta \]with horizontal. A body of mass m rests on it. If the coefficient of friction between the body and the plane is a, then the minimum force that needs to be applied parallel to the inclined plane is
A)
\[\frac{{{p}^{2}}{{t}^{2}}}{3m}\]
done
clear
B)
\[\mu \,mg\sin \theta \]
done
clear
C)
\[\mu \,mgcos\theta +mg\sin \theta \]
done
clear
D)
\[\mu \,mgcos\theta -mg\sin \theta \]
done
clear
View Answer play_arrow
question_answer 16) A particle of mass m at rest is acted upon by a force p for a time t. Its kinetic energy after an interval t is
A)
\[\frac{pt}{2m}\]
done
clear
B)
\[\frac{2m{{L}^{2}}}{3}\]
done
clear
C)
\[\frac{m{{L}^{2}}}{12}\]
done
clear
D)
\[\frac{m{{L}^{2}}}{6}\]
done
clear
View Answer play_arrow
question_answer 17)
A T joint is formed by two identical rods A and B each of mass m and length L in the XY -plane as shown. Its moment of inertia about axis coinciding with A is
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[{{2}^{-3/2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 18) A satellite moves in a circle around th6 earth. The radius of this circle is equal to one-half of the radius of the moons orbit. The satellite completes one revolution is
A)
\[{{2}^{3/2}}\]lunar month
done
clear
B)
\[2:5\]lunar month
done
clear
C)
\[5:2\] lunar month
done
clear
D)
\[2:5\] lunar month
done
clear
View Answer play_arrow
question_answer 19) Two wires of same material and same diameter have lengths in the ratio \[1:3\]. They are stretched by same force. The ratio of work done in stretching them is
A)
\[3:1\]
done
clear
B)
\[\rho \text{ }kg-{{m}^{3}}\]
done
clear
C)
\[10m/{{s}^{2}}\]
done
clear
D)
\[\rho \]
done
clear
View Answer play_arrow
question_answer 20) A bird of mass 1.23 kg is able to hover by imparting a downward velocity of 10 m/s uniformly to air of density \[kg-{{m}^{-3}}\] over an effective area 0.1 m2. If the acceleration due to gravity is \[0.0123\], then the magnitude of \[0.123\]in \[1.23~~\]is
A)
\[1.32\]
done
clear
B)
\[k=200J{{m}^{-1}}-{{K}^{-2}}\]
done
clear
C)
\[{{20}^{o}}C\]
done
clear
D)
\[{{40}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 21) A body of length 1 m having cross-sectional area \[0.75\text{ }{{m}^{2}}\]has heat flow through it at the rate of 6000 J/s. Then find the temperature difference if \[{{80}^{o}}C\]
A)
\[{{100}^{o}}C\]
done
clear
B)
\[{{20}^{o}}C\]
done
clear
C)
\[R=8.31Jmo{{l}^{-1}}{{K}^{-1}}\]
done
clear
D)
\[996\text{ }J\]
done
clear
View Answer play_arrow
question_answer 22) A cylinder of fixed capacity (of 4.48 L) contains 2 moles of helium gas at STP. What is the amount of heat needed to raise the temperature of the gas in the cylinder by \[831\text{ }J\]? (Use \[498\text{ }J\])
A)
\[374\text{ }J\]
done
clear
B)
\[{{25}^{o}}C\]
done
clear
C)
\[{{35}^{o}}C\]
done
clear
D)
\[{{y}_{1}}=0.1\sin \left( 1\omega \pi t+\frac{\pi }{3} \right)\]
done
clear
View Answer play_arrow
question_answer 23) 310 J of heat of required to raise the temperature of 2 moles of an ideal gas at constant pressure from \[{{y}_{2}}=0.1\cos \,100\pi t.\] to \[-\frac{\pi }{3}\]. The amount of heat required to raise the temperature of the gas through the same range at constant volume is
A)
384 J
done
clear
B)
144J
done
clear
C)
276 J
done
clear
D)
452 J
done
clear
View Answer play_arrow
question_answer 24) Two SHMs are represented by the equations \[\frac{\pi }{6}\] and \[-\frac{\pi }{6}\] The phase difference between the speeds of the two paraticles is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[1:2:3\]
done
clear
C)
\[1:4:9\]
done
clear
D)
\[1:3:5\]
done
clear
View Answer play_arrow
question_answer 25) Two simple pendulums whose lengths are 100 cm and 121 cm are suspended side by side. Their bobs are pulled together and then released. After how many minimum oscillations of the longer pendulum, both the pendulums will be in same phase again?
A)
12
done
clear
B)
10
done
clear
C)
22
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 26) A ball is released from the top of a tower. The ratio of work done by force of gravity in first, second and third second of the motion of the ball is
A)
\[1:5:3\]
done
clear
B)
\[{{(Kg)}^{1/2}}\]
done
clear
C)
\[{{(Kg)}^{-1/2}}\]
done
clear
D)
\[{{(Kg)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27) There are two planets. The ratio of radius of the two planets is K but ratio of acceleration due to gravity of both planets is g. What will be the ratio of their escape velocity?
A)
\[{{(Kg)}^{-2}}\]
done
clear
B)
\[\Delta {{\mathbf{p}}_{1}}=-\Delta {{\mathbf{p}}_{2}}\]
done
clear
C)
\[\Delta E=-\Delta (PE+KE)=0\]
done
clear
D)
\[\mathbf{F}\Delta t=m\Delta \mathbf{v}\]
done
clear
View Answer play_arrow
question_answer 28) Consider the following statement. When jumping from some height, you should bend your knees as you come to rest instead of keeping your legs stiff. Which of the following relations can be useful in explaining the statement?
A)
\[\Delta \mathbf{x}\propto \Delta \mathbf{F}\]
done
clear
B)
\[1:8\]
done
clear
C)
\[2\]
done
clear
D)
\[2\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 29) Two rings of radius R and n R made up of same material have the ratio of moment of inertia about an axis passing through centre is \[4\]. The value of n is
A)
\[\frac{1}{2}\]
done
clear
B)
\[60m\]
done
clear
C)
\[50m\]
done
clear
D)
\[30m\]
done
clear
View Answer play_arrow
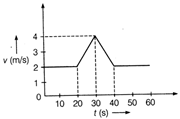
question_answer 30)
Velocity-time (v-t) graph for a moving object is shown in the figure. Total displacement of the object during the time interval when there is non-zero acceleration and retardation is
A)
\[40m\]
done
clear
B)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}r}\]
done
clear
C)
\[\frac{qQ}{4\pi \varepsilon _{0}^{2}{{r}^{2}}}\]
done
clear
D)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}{{r}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 31) Three weights w, 2w and 3w are connected to identical spring suspended from a rigid horizontal rod. The assembly of the rod and the weights fall freely. The positions of the weight from the rod are such that
A)
3 w will be farthest
done
clear
B)
w will be farthest
done
clear
C)
all will be at the same distance
done
clear
D)
2 w will be farthest
done
clear
View Answer play_arrow
question_answer 32) The work done in carrying a charge q once round a circle of radius r with a charge Q at the centre is
A)
\[+1.6\times {{10}^{-16}}C\]
done
clear
B)
\[{{F}_{1}}\]
done
clear
C)
\[{{F}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 33) Two nucleons are at a separation of one fermi. Protons have a charge of \[{{F}_{1}}={{F}_{2}}>{{F}_{3}}\]. The net nuclear force between them is \[{{F}_{1}}={{F}_{2}}={{F}_{3}}\], if both are neutrons, \[{{F}_{1}}<{{F}_{2}}<{{F}_{3}}\] if both are protons and F^ if one is proton and the other is neutron. Then
A)
\[{{F}_{1}}>{{F}_{2}}>{{F}_{3}}\]
done
clear
B)
\[n={{n}_{1}}\]
done
clear
C)
\[n={{n}_{2}}\]
done
clear
D)
\[({{n}_{1}})\]
done
clear
View Answer play_arrow
question_answer 34) The electron in a hydrogen atom makes a transition form \[({{n}_{2}})\] to \[{{n}_{1}}\] state. The time period of the electron in the initial state \[{{n}_{2}}\] is eight times that in the final state \[{{n}_{1}}=8,\,\,\,\,{{n}_{2}}=1\]. The possible values of \[{{n}_{1}}=4,\,\,\,\,{{n}_{2}}=2\] and \[{{n}_{1}}=2,\,\,\,\,{{n}_{2}}=4\] are
A)
\[{{n}_{1}}=1,\,\,\,\,{{n}_{2}}=8\]
done
clear
B)
\[9land\,\,l\]
done
clear
C)
\[~9land\,\,\,\,3l.\]
done
clear
D)
\[5land\,\,l\]
done
clear
View Answer play_arrow
question_answer 35) Two coherent light beams of intensity I and 4I are superposed. The maximum and minimum possible intensities in the .resulting beam are
A)
\[5l\,and\,\,3l\]
done
clear
B)
\[_{0}^{1}n+_{92}^{235}U\to _{92}^{236}U\to _{40}^{98}Zr+_{52}^{136}Te+2_{0}^{1}n\]
done
clear
C)
\[_{42}^{98}{{M}_{0}}\]
done
clear
D)
\[_{54}^{136}X\]
done
clear
View Answer play_arrow
question_answer 36) Calculate the total energy released during a fission reaction \[\beta \] The resulting fission fragments are unstable hence, decay into stable end products \[=1.0087\text{ }amu\] and \[_{92}^{235}U=236.0526amu\]by successive emission of \[_{42}^{98}{{M}_{0}}=97.9054amu\]-particles. Take mass of neutron \[_{54}^{136}Xe=135.9170amu\], mass of \[198MeV\], mass of \[220\text{ }MeV\] and mass of \[185\text{ }MeV\]
A)
\[230\text{ }MeV\]
done
clear
B)
\[{{10}^{-10}}m,\]
done
clear
C)
\[4.2\times {{10}^{15}}\text{ }Hz\]
done
clear
D)
\[0.36\times {{10}^{15}}Hz\]
done
clear
View Answer play_arrow
question_answer 37) If an electron is moving around a nucleus of charge 2e in a circular orbit of radius \[3.6\times {{10}^{15}}Hz\]then calculate the initial frequency of light emitted by the electron
A)
\[4.2\times {{10}^{15}}Hz\]
done
clear
B)
\[4.2\text{ }J/{{g}^{o}}C\]
done
clear
C)
\[3\times {{10}^{9}}Hz\]
done
clear
D)
\[{{20}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 38) Specific heat of water is \[{{40}^{o}}C\]. If light of frequency \[1.69\times {{10}^{29}}\] is used to heat 400 g of water from \[1.69\times {{10}^{28}}\] to \[2.80\times {{10}^{4}}\], the number of moles of photons needed will be
A)
\[2.80\times {{10}^{5}}\]
done
clear
B)
\[{{10}^{4}}\Omega \]
done
clear
C)
\[10\Omega \]
done
clear
D)
\[4.62\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 39) An AC source has an internal resistance of \[2.03\times {{10}^{-2}}\]. Calculate the turn ratio of a transformer so as to match the source to a load of resistance \[3.16\times {{10}^{-2}}\].
A)
\[5.62\text{ }\times {{10}^{-2}}\]
done
clear
B)
\[\lambda \]
done
clear
C)
\[1A\]
done
clear
D)
\[2A\]
done
clear
View Answer play_arrow
question_answer 40) A non-conducting ring of radius r has charge per unit length \[4A\]. A magnetic field perpendicular to plane of the ring changes at rate dB /dt. Torque experienced by the ring is
A)
\[6A\]
done
clear
B)
\[1.33\times {{10}^{-7}}kg\]
done
clear
C)
\[19.8\times {{10}^{-7}}kg\]
done
clear
D)
zero
done
clear
View Answer play_arrow
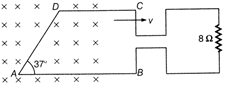
question_answer 41)
The loop ABCD is moving Wight velocity \[9.9\times {{10}^{-7}}kg\]towards right. The magnetic field is 4 T. The loop is connected to a resistance of \[6.6\times {{10}^{-7}}kg\]. If steady current of 2 A flows in the loop, then value of v if loop has a resistance of \[1.1\times {{10}^{-7}}kg\], (Given, AB = 30 cm, AD = 30 cm) is
A)
\[{{10}^{o}}C\]
done
clear
B)
\[{{40}^{o}}C\]
done
clear
C)
\[50s\]
done
clear
D)
\[100s\]
done
clear
View Answer play_arrow
question_answer 42) A length \[150s\] of a wire is bent to form a circular coil of some turns. A current \[200s\] is then established in the coil and it is placed in a uniform magnetic field B. The maximum torque that acts on the coil is
A)
\[4L\]
done
clear
B)
\[2L\]
done
clear
C)
\[\frac{L}{2}\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
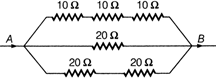
question_answer 43)
The total electrical resistance between the points A and B of the circuit shown is
A)
\[\frac{L}{4}\]
done
clear
B)
\[\propto \text{x}\]
done
clear
C)
\[\propto {{\text{x}}^{2}}\]
done
clear
D)
\[\propto {{\text{x}}^{1/2}}\]
done
clear
View Answer play_arrow
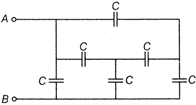
question_answer 44)
The equivalent capacitance between points A and B is
A)
\[p+\frac{1}{2}\rho {{\upsilon }^{2}}+\rho gh=k\]
done
clear
B)
\[\frac{4{{v}^{2}}}{5g}\]
done
clear
C)
\[\frac{4g}{5{{v}^{2}}}\]
done
clear
D)
\[\frac{{{v}^{2}}}{g}\]
done
clear
View Answer play_arrow
question_answer 45) In a region, electric field is parallel to x-axis the equation of equipotential surface is
A)
\[\frac{4{{v}^{2}}}{\sqrt{5}g}\]
done
clear
B)
\[(g=10m/{{s}^{2}})\]
done
clear
C)
\[800N\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 46) In the previous problem, find energy stored in spring at the equilibrium position of the point mass
A)
\[1200N\]
done
clear
B)
\[400N\]
done
clear
C)
\[mg\,sin\,\theta \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 47) For the metallic conductor
A)
dielectric constant must be zero
done
clear
B)
dielectric constant must not be infinity
done
clear
C)
dielectric constant must be infinity
done
clear
D)
dielectric constant may be infinity
done
clear
View Answer play_arrow
question_answer 48) If n coherent sources of intensity \[\frac{{{p}^{2}}{{t}^{2}}}{m}\] are superimposed at a point the intensity of the point is
A)
\[\frac{{{p}^{2}}{{t}^{2}}}{2m}\]
done
clear
B)
\[\frac{{{p}^{2}}{{t}^{2}}}{3m}\]
done
clear
C)
\[\frac{pt}{2m}\]
done
clear
D)
\[\frac{2m{{L}^{2}}}{3}\]
done
clear
View Answer play_arrow
question_answer 49) Find the power and type of the lens by which a person can see clearly the distant objects, if a person cannot see objects beyond 40 cm
A)
- 2.5 D and concave lens
done
clear
B)
- 2.5 D and convex lens
done
clear
C)
- 3.5 D and concave lens
done
clear
D)
- 3.5 D and convex lens
done
clear
View Answer play_arrow
question_answer 50) Surface temperature of the sun as estimated is 6032.25 K. Find the wavelength at which sun radiates maximum energy. (Given, Wiens constant = 0.2898 cm-K)
A)
\[\frac{m{{L}^{2}}}{12}\]
done
clear
B)
\[\frac{m{{L}^{2}}}{6}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[\frac{2}{3}\]
done
clear
View Answer play_arrow
question_answer 51) Conjugate acid of \[{{S}_{2}}O_{8}^{2-}\] is
A)
\[{{H}_{2}}SO_{4}^{-}\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[H{{S}_{2}}O_{8}^{-}\]
done
clear
D)
\[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]
done
clear
View Answer play_arrow
question_answer 52) The pH of a \[1.0\times {{10}^{-8}}M\] solution of \[HCl\] is
A)
\[6\]
done
clear
B)
\[7\]
done
clear
C)
\[8\]
done
clear
D)
\[6.98\]
done
clear
View Answer play_arrow
question_answer 53) The common components of photochemica smog are ozone, nitric oxide, acrole in formaldehyde and peroxyacetylnitrate Photochemical smog cause
A)
cancer
done
clear
B)
cracking of rubber
done
clear
C)
oxygen deficiency in blood
done
clear
D)
global warming
done
clear
View Answer play_arrow
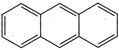
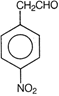
question_answer 54) Which of the following compound is aromatic?
A)
done
clear
B)
done
clear
C)
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 55) The IUPAC name of the compound \[CNC{{H}_{2}}CHCNC{{H}_{2}}CN\] is
A)
1,2,3-tricyanopropane
done
clear
B)
1,2,3-cyanopropane
done
clear
C)
1,2,3-propane trinitrile
done
clear
D)
3-cyano-1,5-dinitrile
done
clear
View Answer play_arrow
question_answer 56) Which of the following is not a chiral?
A)
2-butanol
done
clear
B)
2,3-dibromopentane
done
clear
C)
3-bromopentane
done
clear
D)
2-hydroxy propanoic acid
done
clear
View Answer play_arrow
question_answer 57) The \[pOH\] of an ammonia solution is 4.75. The pH of solution formed on mixing \[0.2M\] \[N{{H}_{4}}Cl\] and \[0.1M\] \[N{{H}_{3}}\]will be
A)
\[7.00\]
done
clear
B)
\[7.85\]
done
clear
C)
\[8.00\]
done
clear
D)
\[8.95\]
done
clear
View Answer play_arrow
question_answer 58) For redox reaction,\[F{{e}^{2+}}(aq)+C{{r}_{2}}O_{7}^{2-}(aq)+{{H}^{+}}(aq)\xrightarrow{{}}\] \[F{{e}^{3+}}(aq)+C{{r}^{3+}}(aq)+{{H}_{2}}O(l)\] The coefficients of \[F{{e}^{2+}},C{{r}_{2}}O_{7}^{2-}\] and \[{{H}^{+}}\] in the balanced reaction are
A)
\[6,1,14\]
done
clear
B)
\[3,2,6\]
done
clear
C)
\[2,3,6\]
done
clear
D)
\[6,4,12\]
done
clear
View Answer play_arrow
question_answer 59) Outer electronic configuration of phosphorus is \[3{{s}^{2}}3{{p}^{3}}\], however it cannot form \[P{{H}_{5}}\] because
A)
high \[{{\Delta }_{a}}H\] value of dihydrogen do not favour the formation of \[P{{H}_{5}}\]
done
clear
B)
\[{{\Delta }_{eg}}H\] value of hydrogen do not favour the formation of \[P{{H}_{5}}\]
done
clear
C)
Both (a) and (b) are correct
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 60) When lithium nitrate is heated
A)
only \[L{{i}_{2}}O\] is formed
done
clear
B)
only \[{{O}_{2}}\] is formed
done
clear
C)
only \[N{{O}_{2}}\] is formed
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 61) White lead is
A)
\[PbO\]
done
clear
B)
\[P{{b}_{3}}{{O}_{4}}\]
done
clear
C)
\[2PbC{{O}_{3}}.Pb{{(OH)}_{2}}\]
done
clear
D)
\[Pb{{(C{{H}_{3}}COO)}_{2}}.Pb{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 62) Electronic configuration of hydride ion is
A)
\[1{{s}^{0}}\]
done
clear
B)
\[1{{s}^{1}}\]
done
clear
C)
\[1{{s}^{2}}\]
done
clear
D)
\[1{{s}^{1}}.2{{s}^{1}}\]
done
clear
View Answer play_arrow
question_answer 63) What is the wavelength of a photon emitted during a transition from \[n=5\]state to \[n=u~\] state in the hydrogen atom?
A)
\[400nm\]
done
clear
B)
\[421nm\]
done
clear
C)
\[434nm\]
done
clear
D)
\[43.4nm\]
done
clear
View Answer play_arrow
question_answer 64) The number of molecules in 44 g of \[C{{O}_{2}}\] in terms of Avogadro number \[{{N}_{A}}\] is
A)
\[10{{N}_{A}}\]
done
clear
B)
\[{{10}^{-1}}{{N}_{A}}\]
done
clear
C)
\[{{10}^{-2}}{{N}_{A}}\]
done
clear
D)
\[{{10}^{-3}}{{N}_{A}}\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following species has a linear shape?
A)
\[{{O}_{3}}\]
done
clear
B)
\[NO_{2}^{-}\]
done
clear
C)
\[NO_{2}^{+}\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) Hydrogen bond is not present in
A)
methanol
done
clear
B)
glycerol
done
clear
C)
water
done
clear
D)
hydrogen sulphide
done
clear
View Answer play_arrow
question_answer 67) A, B and C are ideal gases. Their molecular weights are 2, 4 and 20 respectively. The rate of diffusion of these gases follow the order
A)
\[A>B>C\]
done
clear
B)
\[A=B=C\]
done
clear
C)
\[C>B>A\]
done
clear
D)
\[A>C>B\]
done
clear
View Answer play_arrow
question_answer 68) For the reaction, \[2Cl(g)\xrightarrow{{}}C{{l}_{2}}(g),\] the signs of \[\Delta H\] and \[\Delta S\] are
A)
\[\Delta H=+ve,\,\Delta S=-ve\]
done
clear
B)
\[\Delta H=-ve,\,\Delta S=+ve\]
done
clear
C)
\[\Delta H=+ve,\,\Delta S=+ve\]
done
clear
D)
\[\Delta H=-ve,\,\Delta S=-ve\]
done
clear
View Answer play_arrow
question_answer 69) One mole of ethanol when burnt in oxygen, gives out a: kJ \[mo{{l}^{-1}}\] heat. If one mole of \[{{O}_{2}}\] is used, what will be the amount of heat evolved?
A)
\[x\,kJ\]
done
clear
B)
\[\frac{x}{3}\,kJ\]
done
clear
C)
\[3x\,kJ\]
done
clear
D)
\[\frac{3x}{2}\,kJ\]
done
clear
View Answer play_arrow
question_answer 70) The correct order in which the first ionisation potential increases is
A)
\[Na,Li,B,Be\]
done
clear
B)
\[Li,Na,Be,B\]
done
clear
C)
\[Li,Be,B,Na\]
done
clear
D)
\[Na,Li,Be,B\]
done
clear
View Answer play_arrow
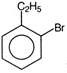
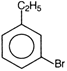
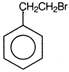
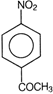
question_answer 71) Ethyl benzene with bromine in the presence of \[FeB{{r}_{3}}\], predominantly gives
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 72) Density of Li atom is \[0.53g\,\,c{{m}^{-3}}\]. The edge length of \[Li\] is\[3.5\overset{\text{o}}{\mathop{\text{A}}}\,\]. Find out the number of \[Li\]atoms in a unit cell \[({{N}_{A}}=6.023\times {{10}^{23}},\,M=6.94)\]
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 73) An aqueous solution containing 1.248 g of barium chloride (molar mass \[=208.34g\,mo{{l}^{-1}}\]) in 100 g of water boils at\[{{100.0832}^{o}}C\]. The degree of dissociation of \[BaC{{l}_{2}}\] will be (\[{{K}_{b}}\] for water\[=0.52K\text{ }kg\text{ }mo{{l}^{-1}}\])
A)
\[0.835\]
done
clear
B)
\[0.756\]
done
clear
C)
\[0.638\]
done
clear
D)
\[0.930\]
done
clear
View Answer play_arrow
question_answer 74) On a certain hill station, pure water is found to boil at \[{{95}^{o}}C\]. How many grams of \[NaCl\] must be added to 2 kg of water so that it boil at \[{{100}^{o}}C\]?
A)
\[125.0g\]
done
clear
B)
\[206.85\text{ }g\]
done
clear
C)
\[346.70\text{ }g\]
done
clear
D)
\[562.42\text{ }g\]
done
clear
View Answer play_arrow
question_answer 75) On dissolving sugar in water at room temperature, solution feels cool to touch. Under which of the following cases dissolution of sugar will be most rapid?
A)
Sugar crystals in cold water
done
clear
B)
Sugar crystals in hot water
done
clear
C)
Powdered sugar in cold water
done
clear
D)
Powdered sugar in hot water
done
clear
View Answer play_arrow
question_answer 76) Which of the following solution has the highest equivalent conductance?
A)
\[0.01M\text{ }KCl\]
done
clear
B)
\[0.05M\text{ }KCl\]
done
clear
C)
\[0.02M\text{ }KCl\]
done
clear
D)
\[0.005M\text{ }KCl\]
done
clear
View Answer play_arrow
question_answer 77) Among the following cells, Leclanche cell (I), nickel-cadmium cell (II), lead storage battery (III), mercury cell (IV), primary cells are
A)
I and II
done
clear
B)
I and III
done
clear
C)
II and III
done
clear
D)
I and IV
done
clear
View Answer play_arrow
question_answer 78) The function of gum arable in the preparation Indian ink is
A)
coagulation
done
clear
B)
adsorption
done
clear
C)
peptisation
done
clear
D)
protective action
done
clear
View Answer play_arrow
question_answer 79) During smelting, an additional substance is added which combines with impurities to form a fusible mass. The additional substance is called
A)
ore
done
clear
B)
gangue
done
clear
C)
flux
done
clear
D)
slag
done
clear
View Answer play_arrow
question_answer 80) Which of the following statement is incorrect about collision theory of a chemical reaction?
A)
It considers reacting molecules or atoms to be hard spheres and ignores their structural features
done
clear
B)
Number of effective collisions determines the rate of reaction
done
clear
C)
Collisions of atoms or molecules possessing sufficient threshold energy results into the product formation
done
clear
D)
Molecules should collide with sufficient threshold energy and proper orientation for the collision to be effective
done
clear
View Answer play_arrow
question_answer 81) Zinc/silver oxide cell is used in hearing acids and electric watches. The following reactions takes place \[Zn\xrightarrow{{}}Z{{n}^{2+}}+2{{e}^{-}};{{E}^{o}}=0.76V\]\[A{{g}_{2}}O+{{H}_{2}}O+2{{e}^{-}}\xrightarrow{{}}2Ag+2O{{H}^{-}};\]\[{{E}^{o}}=0.344V\]\[\Delta G\] in joules will be
A)
\[-2.13\times {{10}^{5}}J\]
done
clear
B)
\[+2.13\times {{10}^{5}}J\]
done
clear
C)
\[-4.67\times {{10}^{7}}J\]
done
clear
D)
\[+4.67\times {{10}^{7}}J\]
done
clear
View Answer play_arrow
question_answer 82) Which one of the following is a preservative?
A)
Sodium stearate
done
clear
B)
Sodium benzoate
done
clear
C)
Sodium sulphate
done
clear
D)
Sodium thiosulphate
done
clear
View Answer play_arrow
question_answer 83) Which one of the following polymer is used for making contact lenses?
A)
Polyacrylonitrile
done
clear
B)
Polymethylmethacrylate
done
clear
C)
Neoprene
done
clear
D)
Dacron
done
clear
View Answer play_arrow
question_answer 84) Which one of the following is not an aldose?
A)
Glucose
done
clear
B)
Ribose
done
clear
C)
Fructose
done
clear
D)
Mannose
done
clear
View Answer play_arrow
question_answer 85) The hormone which controls the processes of burning of fats, proteins and carbohydrates and liberates energy in the body is
A)
thyroxine
done
clear
B)
adrenaline
done
clear
C)
cortisone
done
clear
D)
insulin
done
clear
View Answer play_arrow
question_answer 86) DNA and RNA contain four bases each. Which of the following bases is not present in DNA?
A)
Adenine
done
clear
B)
Uracil
done
clear
C)
Thymine
done
clear
D)
Cytosine
done
clear
View Answer play_arrow
question_answer 87) Benzyl amine may be alkylated as shown in the following equation\[{{C}_{6}}{{H}_{6}}C{{H}_{2}}N{{H}_{2}}+R-X\xrightarrow{{}}{{C}_{6}}{{H}_{5}}C{{H}_{2}}NHR.\] Which of the following alkyl halides is best suited for this reaction through \[{{S}_{N}}1\] mechanism?
A)
\[C{{H}_{3}}Br\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}Br\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}Br\]
done
clear
View Answer play_arrow
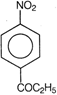
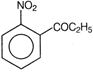
question_answer 88) Which one of the following is the structure of 4-nitropropiophenone?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 89) Diethyl ketone \[(C{{H}_{3}}C{{H}_{2}}COC{{H}_{2}}C{{H}_{3}})\] and acetone can be distinguished by
A)
Tollenstest
done
clear
B)
sodium bisulphite tes
done
clear
C)
sodium test
done
clear
D)
\[FeC{{l}_{3}}\] test
done
clear
View Answer play_arrow
question_answer 90) In the following reaction, reagent X will be\[{{C}_{6}}{{H}_{5}}COONa\xrightarrow{X,\Delta }{{C}_{6}}{{H}_{6}}\]
A)
soda lime
done
clear
B)
\[NaOH\]
done
clear
C)
\[KMn{{O}_{4}}\]
done
clear
D)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 91) Phenol \[\xrightarrow{X}\] forms a tribromo derivative. X is
A)
bromine in benzene
done
clear
B)
bromine in water
done
clear
C)
\[KBr\] solution
done
clear
D)
\[B{{r}_{2}}\] in \[CC{{l}_{4}}\] at \[{{0}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 92) \[C{{H}_{3}}O{{C}_{2}}{{H}_{5}}\] and \[{{(C{{H}_{3}})}_{3}}COC{{H}_{3}}\] are treated with \[HI\]. The fragments after reaction obtained are
A)
\[C{{H}_{3}}l+{{C}_{2}}{{H}_{5}}OH;\,{{(C{{H}_{3}})}_{3}}C-l+C{{H}_{3}}OH\]
done
clear
B)
\[C{{H}_{3}}OH+{{C}_{2}}{{H}_{5}}l;\,{{(C{{H}_{3}})}_{3}}C-OH+C{{H}_{2}}l\]
done
clear
C)
\[C{{H}_{3}}l+{{C}_{2}}{{H}_{5}}OH;\,C{{H}_{3}}l+{{(C{{H}_{3}})}_{3}}C-OH\]
done
clear
D)
\[C{{H}_{3}}OH+{{C}_{2}}{{H}_{5}}l;{{(C{{H}_{3}})}_{3}}C-l+C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 93) Chloromethane on treatment with excess of \[N{{H}_{3}}\] yields mainly
A)
N,N-dimethylmethanamine
done
clear
B)
N-methylmethanamine
done
clear
C)
methanamine
done
clear
D)
mixture containing all these in equal proportion
done
clear
View Answer play_arrow
question_answer 94) The most stable complex among the following is
A)
\[{{K}_{3}}[Al{{({{C}_{2}}{{O}_{4}})}_{3}}]\]
done
clear
B)
\[[Ag{{(N{{H}_{3}})}_{2}}]Cl\]
done
clear
C)
\[{{K}_{2}}[Ni(EDTA)]\]
done
clear
D)
\[[Pt{{(en)}_{2}}]C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 95) Formation of interstitial compounds makes the transition metal
A)
more soft
done
clear
B)
more ductile
done
clear
C)
more metallic
done
clear
D)
more hard and brittle
done
clear
View Answer play_arrow
question_answer 96) Although Zr belongs to 4rf-transition series and Hf to 5rf-transition series even then they show similar physical and chemical properties because
A)
both have same number of electrons
done
clear
B)
both have similar atomic radius
done
clear
C)
both belong to d-block
done
clear
D)
both belong to the same group of the periodic table
done
clear
View Answer play_arrow
question_answer 97) Which one of the following orders are not correct as per the properties mentioned against each?
A)
\[{{F}_{2}}>C{{l}_{2}}>B{{r}_{2}}>{{I}_{2}}\] Bond dissociation enthalpy
done
clear
B)
\[{{H}_{2}}O>{{H}_{2}}S>{{H}_{2}}Se>{{H}_{2}}Te\] Thermal stability
done
clear
C)
\[S{{O}_{2}}>{{P}_{2}}{{O}_{3}}>A{{s}_{2}}{{O}_{3}}>Si{{O}_{2}}\] Acid strength
done
clear
D)
\[F>Cl>O>S\] More negative electron gain enthalpy
done
clear
View Answer play_arrow
question_answer 98) Which one of the following statement is wrong about \[Cl{{O}_{2}}\]?
A)
Central atom \[Cl\] is \[s{{p}^{2}}\] hybridized
done
clear
B)
\[O-Cl-O\]bond angle is 118°
done
clear
C)
\[Cl-O\] bond has appreciable double bond character due to \[p\pi -d\pi \] bonding
done
clear
D)
It is diamagnetic
done
clear
View Answer play_arrow
question_answer 99) Number of lone pairs of electrons on \[Xe\] atoms in \[Xe{{F}_{2}}\], \[Xe{{F}_{4}}\], \[Xe{{F}_{6}}\] and \[Xe{{O}_{4}}\] molecules are respectively
A)
\[3,2,\text{ }1,0\]
done
clear
B)
\[1,3,2,0\]
done
clear
C)
\[0,2,3,1\]
done
clear
D)
\[3,2,0,1\]
done
clear
View Answer play_arrow
question_answer 100) Treatment of hydroxyl amine \[(N{{H}_{2}}OH)\] with an excess of \[Fe(III)\]results in the formation of \[NgO\] and an equivalent amount of \[Fe(II)\]. \[2N{{H}_{2}}OH+4F{{e}^{3+}}\xrightarrow{{}}{{N}_{2}}O(g)+4F{{e}^{2+}}\]\[+4{{H}^{+}}+{{H}_{2}}O\]Calculate the molar concentration of an \[N{{H}_{2}}OH\]solution if \[Fe(II)\] produced from 50 mL solution required 23.61 mL of \[0.0217M\] \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
A)
\[0.0307\text{ }M\]
done
clear
B)
\[0.0316M\]
done
clear
C)
\[0.1431\text{ }M\]
done
clear
D)
\[0.1448M\]
done
clear
View Answer play_arrow
question_answer 101) Which of the following animal has become extinct after death of last representative in 1994 in Delhi zoo?
A)
Raphus cuculatus
done
clear
B)
Panthera leopersica
done
clear
C)
Macaca silenus
done
clear
D)
Cheetah
done
clear
View Answer play_arrow
question_answer 102) A person is smoking in a public place. What is added by him in environment?
A)
Carbon dioxide
done
clear
B)
Carbon monoxide
done
clear
C)
Smoking causes pollution
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 103) Unloading of oxygen from haemoglobin at the site of diffusion is facillated by
A)
higher pH of blood
done
clear
B)
partial pressure of \[{{O}_{2}}\] and \[C{{O}_{2}}\]
done
clear
C)
low temperature in lungs and higher temperature in diffusion
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 104) Blood does not transport oxygen in
A)
earthworm
done
clear
B)
pigeon
done
clear
C)
cockroach
done
clear
D)
hirudinaria
done
clear
View Answer play_arrow
question_answer 105) Diphtheria is caused by
A)
poisons released by a living bacterial cells into host tissues
done
clear
B)
poisons released by a dead bacterial cells into host tissues
done
clear
C)
poisons released by virus into host tissues
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 106) The nature of sebaceous gland, present in dermis of magority of the mammals are
A)
holocrine
done
clear
B)
apocrine
done
clear
C)
merocrine
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 107) Mammary glands are modified form of
A)
sebaceous gland
done
clear
B)
sweat gland
done
clear
C)
meibomian glands
done
clear
D)
ceruminous glands
done
clear
View Answer play_arrow
question_answer 108) The most abundant minerals in body of vertebrates are
A)
\[Ca\]
done
clear
B)
\[K\] and \[Cl\]
done
clear
C)
\[{{K}^{+}}\]and \[P{{O}_{4}}\]
done
clear
D)
\[Ca\] and P
done
clear
View Answer play_arrow
question_answer 109) Choose the correct statements from the followings
A)
Tsar or Tussor silk is obtained by Bombay mori
done
clear
B)
Wucheria lbacrofti is digenetic nematodes viviparous
done
clear
C)
Leishmania a protozoan causes African sleeping sickness
done
clear
D)
World heart day is celebrated on last Sunday of October
done
clear
View Answer play_arrow
question_answer 110) Which one is/are a living fossils as well as a connecting link between pisces and amphibians?
A)
Tuatara
done
clear
B)
Lung fish
done
clear
C)
Latimeria
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 111) Foramen obturator is present between
A)
ileum and ischium
done
clear
B)
ischium and pubis
done
clear
C)
ischium and sacrum
done
clear
D)
ileum and pubis
done
clear
View Answer play_arrow
question_answer 112) Who has proposed the sol gel theory for amoeboid movement explanation?
A)
Pantin
done
clear
B)
Goldacre and love
done
clear
C)
Hyman
done
clear
D)
Mast
done
clear
View Answer play_arrow
question_answer 113)
Match correctly the entities of column with column II and choose the correct option from codes given below. Column I Column II A. \[{{M}_{13}}\] bacteriophage 1. d/s RNA B. Rice dwarf virus 2. ss RNA C. Cauliflower mosaic Virus 3. Ss DNA D. Polio Virus 4. ds DNA
Codes
A)
A-3 A-1 A-4 A-2
done
clear
B)
B-1 B-3 B-4 B-2
done
clear
C)
C-3 C-4 C-1 C-2
done
clear
D)
C-3 C-1 C-2 C-4
done
clear
View Answer play_arrow
question_answer 114) Which one is a nuisance for swimmer/bather and belong to class-Hydrozoa?
A)
Aurelia
done
clear
B)
Physalia
done
clear
C)
Halistema
done
clear
D)
Obelia
done
clear
View Answer play_arrow
question_answer 115) Which one set is match correctly regarding phylum class and example?
A)
Protozoa-Mastigophora-Entemoeba
done
clear
B)
Arthopoda-Diplopoda-Scolopendra
done
clear
C)
Chordata -Cyclostoma-phrynosoma
done
clear
D)
Mollusca-Bivalvia-Pinctada
done
clear
View Answer play_arrow
question_answer 116)
Match the following with reference to common earthworm Column I Column II A. Nephridiophores 1. 26th to 95th segments B. Exonephric nephridia 2. 10th segment C. Stomach 3. 3rd segment to last. Segment D. Testes 4. 200-250 E. Typhlosole 5. 9th to 14th segment
Codes
A)
A-3 A-4 A-5 A-2 A-1
done
clear
B)
B-4 B-3 B-5 B-2 B-1
done
clear
C)
C-3 C-4 C-1 C-2 C-5
done
clear
D)
D-3 D-4 D-5 D-2 D-1
done
clear
View Answer play_arrow
question_answer 117) 2n the life cycle (asexual) of Plasmodium 7, 2nd and 3rd phase takes places in
A)
RBCs of man
done
clear
B)
Liver and RBCs of host
done
clear
C)
Liver of the host
done
clear
D)
Alimentry canal of mosquito
done
clear
View Answer play_arrow
question_answer 118) Largest protozoan fossil is .......... a fossil foraminifer, and species Entamoeba histolytica was established by .........
A)
Noctiluca and Leidy (1879)
done
clear
B)
Noctiluca and Jenning (1904)
done
clear
C)
Nummulites and Schaudinn (1903)
done
clear
D)
Nummulites and L-Muller
done
clear
View Answer play_arrow
question_answer 119)
Go through the following statement regarding invertebrates. Choose true and false and answer appropriate option. I. Germ layers are always three in higher invertebrates. II. Leidy (1879) described Amoeba proteus. III. Amoeba is said to naked because it has no body covering.
A)
All are true
done
clear
B)
All statement are false
done
clear
C)
Statement I and III are true
done
clear
D)
Statement I and II and true
done
clear
View Answer play_arrow
question_answer 120) Occurance of an algae in body wall of Hydra and of the cellulose digesting protozoans in termites gut is an example of
A)
A food chain involving a parasite
done
clear
B)
predation
done
clear
C)
Commensalism
done
clear
D)
Mutualism
done
clear
View Answer play_arrow
question_answer 121) Open type of nephridia in south Indian earthwarm are
A)
mega
done
clear
B)
micro
done
clear
C)
pharyngeal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 122) Ants belong to order
A)
Lepidoptera
done
clear
B)
Rhizopoda
done
clear
C)
Arthropoda
done
clear
D)
Hymenoptera
done
clear
View Answer play_arrow
question_answer 123) The rate of erythropoiesis is controlled by
A)
an enzyme
done
clear
B)
a hormone
done
clear
C)
hypothalamys
done
clear
D)
spleen
done
clear
View Answer play_arrow
question_answer 124) A bone is distinguished from cartilage by the presence of
A)
blood vessels
done
clear
B)
lymph vessels
done
clear
C)
haverisian canals
done
clear
D)
collagen proteins
done
clear
View Answer play_arrow
question_answer 125) Bone of patella is an example of
A)
cartilagenous bone
done
clear
B)
dermal bone
done
clear
C)
spongy bone
done
clear
D)
sesamoid bone
done
clear
View Answer play_arrow
question_answer 126) Which cell organelle show polymorphism?
A)
Lysosomes
done
clear
B)
Ribosomes
done
clear
C)
Peroxisomes
done
clear
D)
Endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 127) Animal cell lacking nucleus would also lack in
A)
chromosomes
done
clear
B)
ribosomes
done
clear
C)
centrioles
done
clear
D)
lysosomes
done
clear
View Answer play_arrow
question_answer 128) Which one of the cell organelle does not contain its own DNA, however is able to replicate?
A)
Ribosomes
done
clear
B)
Centrioles
done
clear
C)
Nucleolus
done
clear
D)
Peroxysomes
done
clear
View Answer play_arrow
question_answer 129) Which of the two enzyme are used in laundry and kitchen to wash clothes and dishes respectively?
A)
Lipases and proteases
done
clear
B)
Amylase and lipase
done
clear
C)
Proteases and amylases
done
clear
D)
Proteases and lipases
done
clear
View Answer play_arrow
question_answer 130) Protective structure like nails hoofs, hairs and horns in mammals are produced in and derivative of
A)
stratum granulosum and epidermis
done
clear
B)
stratum corneum and epidermal derivatives
done
clear
C)
stratum Malpighi and epidermis
done
clear
D)
stratum lucidum and epidermal derivatives
done
clear
View Answer play_arrow
question_answer 131) Cyclosporin and endosporin are drugs which are used as
A)
immuno modulators
done
clear
B)
anti-retroviral drugs
done
clear
C)
immuo-suppresants
done
clear
D)
Immuno vaccines
done
clear
View Answer play_arrow
question_answer 132) How antigen is bound to antibody?
A)
By electrostatic interaction
done
clear
B)
By civalent bonds
done
clear
C)
By disulphide bridge
done
clear
D)
By amide formation
done
clear
View Answer play_arrow
question_answer 133) Which is the correct sequence in development of human being?
A)
Fertilisation, zygote cleavage, morula, gastrula
done
clear
B)
Fertilisation, zygote cleavage, morula, blastula gastrula
done
clear
C)
Zygote, morula, blastula, cell differentiation
done
clear
D)
Fertilisation, cleavage, gastrula, morula blastula
done
clear
View Answer play_arrow
question_answer 134) How many secondary spermatocytes will form 400 spermatoza?
A)
400
done
clear
B)
40
done
clear
C)
200
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 135) From which layer of embryo do the liver and pancreas develop?
A)
Ectoderm
done
clear
B)
Mesoderm
done
clear
C)
Endoderm
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 136) Which animals (mammals) contain more amount of yolk then cytoplasm in their eggs?
A)
Placenta! mammals
done
clear
B)
Aquatic mammals
done
clear
C)
All eutherians
done
clear
D)
Prototherians
done
clear
View Answer play_arrow
question_answer 137) How morula is distinguished from blastula?
A)
Absence of yolk
done
clear
B)
Presence of cavity
done
clear
C)
Absence of cavity
done
clear
D)
Presence of more amount of yolk
done
clear
View Answer play_arrow
question_answer 138) The branch of zoology which is associated with the studies of abnormal embroyonic development is known as
A)
amniocentasis
done
clear
B)
teratology
done
clear
C)
embryology
done
clear
D)
gerontology
done
clear
View Answer play_arrow
question_answer 139) Which one of the following bone is also said to be a funny bone?
A)
Humerus
done
clear
B)
Radius
done
clear
C)
Ulna
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 140) Which type of synovial joint is absent in frog but is responsible for movement of thumb in many direct in mammals?
A)
Pivot joint
done
clear
B)
Saddle joint
done
clear
C)
Ball and socket joint
done
clear
D)
Condyloid joint
done
clear
View Answer play_arrow
question_answer 141) Long neck of camel is because of
A)
muscle between two vertebrae
done
clear
B)
increase in length of cervical vertebrae,
done
clear
C)
bony plate between two vertebrae
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 142) A couple is normal vision but their parents were colour blind. What is the probability of their first daughter to be colour blind?
A)
25%
done
clear
B)
50%
done
clear
C)
75%
done
clear
D)
0%
done
clear
View Answer play_arrow
question_answer 143) A person meet with a severe road accident and immediately is in need of blood transfusion. But there is no time to analyse the blood group of victim which is the safe transfer blood of group?
A)
\[OR\overline{h}\]
done
clear
B)
\[O.R{{h}^{+}}\]
done
clear
C)
Both (a) and (b)
done
clear
D)
AB. \[R{{h}^{+}}\]
done
clear
View Answer play_arrow
question_answer 144) Detection of blood group is done by agglutinisation test using antiserum, according to the test
A)
If the blood shows coagulation with antiserum B, the blood group is B
done
clear
B)
If the blood show coagulation with both antiserum A and B, the blood group is O
done
clear
C)
If the blood show coagulation with antiserum A the blood group is AB
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 145) Who was/were the pioneer worker/workers on polygenic inheritance?
A)
SK Davenport on skin colour in man
done
clear
B)
Joseph Kolreutar on tobacco plant
done
clear
C)
F Galton on human being
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 146) Multiple allelism control inheritance of
A)
sickle-cell anaemia
done
clear
B)
colour blind
done
clear
C)
blood group
done
clear
D)
phenylketonuria
done
clear
View Answer play_arrow
question_answer 147) Darwin theory of Natural selection was based upon
A)
Malthus idea of population
done
clear
B)
Mutations
done
clear
C)
Struggle for existence
done
clear
D)
Inheritance of acquired characters
done
clear
View Answer play_arrow
question_answer 148) Lungs of rabbit and gills of rohu are example of
A)
analogous organ
done
clear
B)
homologous organ
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 149) Species occuring in different geographical areas are called as
A)
sibling
done
clear
B)
sympatric
done
clear
C)
allopatric
done
clear
D)
neopatric
done
clear
View Answer play_arrow
question_answer 150) Three bones scapula, clavicle and coracoid enclose and meet at
A)
foramen of triosseum
done
clear
B)
foramen of rotunda
done
clear
C)
foramen of spinosum
done
clear
D)
foramen obturator
done
clear
View Answer play_arrow
question_answer 151) Fluid mosaic model of plasma membrane was proposed by
A)
SJ Singer and GL Nicholson 1972
done
clear
B)
SK Singer and JL Nicholson in 1982
done
clear
C)
G Nicholson and N Daniel 1892
done
clear
D)
SJ Singer and N Daniel in 1972
done
clear
View Answer play_arrow
question_answer 152) What is HLA?
A)
Group of polypeptides
done
clear
B)
Group of genes
done
clear
C)
Group of chromosomes
done
clear
D)
Group of proteins
done
clear
View Answer play_arrow
question_answer 153) \[{{C}_{{{4}^{-}}}}\]plants show an advantage over \[{{C}_{{{3}^{-}}}}\]-plants when the whether is
A)
dry
done
clear
B)
cold and dry
done
clear
C)
hot and dry
done
clear
D)
hot and wet
done
clear
View Answer play_arrow
question_answer 154) Black foot disease is caused by contaminated ground water with excess of
A)
fluoride
done
clear
B)
arsenic
done
clear
C)
cadmium
done
clear
D)
mercury
done
clear
View Answer play_arrow
question_answer 155) Who is regarded as father of Indian Bryology?
A)
KC Mehta
done
clear
B)
VK Kashyap
done
clear
C)
SR Kashyap
done
clear
D)
RN Singh
done
clear
View Answer play_arrow
question_answer 156) Select a set of correct matching.
A)
SAWaksman - Discover first antibiotics effective against tuberculosis
done
clear
B)
Ochoa and - They got Noble Prized for Kornberg giving structure of RNA
done
clear
C)
Nirenberg - In vitro synthesis of nucleic acid
done
clear
D)
Fisher and - Noble Prize in peace for Krebs developing for developing disease resistant varities of wheat
done
clear
View Answer play_arrow
question_answer 157) Amphibious hydrophyte are
A)
Helcephytes
done
clear
B)
Limnophytes
done
clear
C)
Helophytes
done
clear
D)
Halophytes
done
clear
View Answer play_arrow
question_answer 158)
Match entities of column I with column II correctly. Column I Column II A. George Button 1. Snakes of India B. Archibald Garrod 2. Natural History C. PJ Deoras 3. Tigor! Tigor! D. Kailash Sankhala 4. In born error of metabolism
Codes
A)
A-2 A-4 A-3 A-1
done
clear
B)
B-2 B-4 B-1 B-3
done
clear
C)
C-2 C-3 C-4 C-1
done
clear
D)
D-2 D-1 D-4 D-3
done
clear
View Answer play_arrow
question_answer 159) Which is a source of anticancer drug?
A)
Tagetes
done
clear
B)
Tamarix
done
clear
C)
Thea
done
clear
D)
Taxus
done
clear
View Answer play_arrow
question_answer 160) Term (hybrid vigour) was coined by
A)
Boweri
done
clear
B)
MS Swaminathan
done
clear
C)
GSShull
done
clear
D)
Barbara McClintock
done
clear
View Answer play_arrow
question_answer 161) Which of the following taxonomist described classification of plant in families of flowering plant?
A)
Eichler
done
clear
B)
Hutchinson
done
clear
C)
Thakhtagan
done
clear
D)
Benson
done
clear
View Answer play_arrow
question_answer 162) A free living nitrogen fixing blue-green algae which also form a symbiotic association with a pteridophyte
A)
Anabaena
done
clear
B)
Chlorella
done
clear
C)
Nostoc
done
clear
D)
Tolypothrix
done
clear
View Answer play_arrow
question_answer 163) Which one of the following statement define accurately can plasmid?
A)
Bacteria
done
clear
B)
Extra chromosomal DNA in bacteria
done
clear
C)
An extrachromosomal DNA is bacterial cell which can replicate independently
done
clear
D)
occur in bacteria and can replicate independently
done
clear
View Answer play_arrow
question_answer 164) Which pigment is responsible for photoperiodic change in plants?
A)
Chlorophyll
done
clear
B)
Phytochrome
done
clear
C)
Cytochrome
done
clear
D)
Anthocynin
done
clear
View Answer play_arrow
question_answer 165) Opening of flower buds into flower is a type of
A)
Nastic movement
done
clear
B)
Hyponasty movement
done
clear
C)
Epinastic movement
done
clear
D)
Autonomic movement of growth
done
clear
View Answer play_arrow
question_answer 166) The pollen grains are librated in Cassytha by
A)
Poruous dehiscence
done
clear
B)
Longitudinal dehiscence
done
clear
C)
Transverse dehiscence
done
clear
D)
Valvular dehiscence
done
clear
View Answer play_arrow
question_answer 167)
Identify the characters with reference for the plant in which eight nucleated embryo sac was first studied by Strasburger I. Micropyle, chalaza and funiculus are arranged in same vertical line in a ovule II. Presence of both unisexual and bisexual flowers in same plant. III. Fill form apparatus facilitates conduction of food from endosperm to egg apparatus.
A)
I and II
done
clear
B)
Only I
done
clear
C)
II and III
done
clear
D)
Only II
done
clear
View Answer play_arrow
question_answer 168) Go through the following statements regarding a gymnosperm Pinus. Choose characteristic when does not occur in genus
A)
The Pinus is homosporous gymnosperm, i.e., produce same type of spores.
done
clear
B)
The number of needles in spur of Pinus roxburghii is three
done
clear
C)
Each vascular bundle in the long shoot of Pinus consists of xylem facing towards the centre of the shoot
done
clear
D)
Microsporophyll of Pinus bear two micro sporangia
done
clear
View Answer play_arrow
question_answer 169) Which one of the following set of genera belong to same class?
A)
Chara, Fucus, Polysiphonia
done
clear
B)
Porphyra, Ectocarpus, Ulothrix
done
clear
C)
Sargassum, Laminaria, Geliduim
done
clear
D)
Volvox, Spirogyra, Chlamydomonas
done
clear
View Answer play_arrow
question_answer 170) Given below statements are concerned with sporophyte of a non-vascular plant. Choose correct statement
A)
Sporophyte is partially parasite on gametophyte
done
clear
B)
Sporophyte produces spore/gametes that give rise gametophyte
done
clear
C)
It arises form a spore produced from the gametophyte
done
clear
D)
It manufacture/synthesis food for itself as well for the gametophytes
done
clear
View Answer play_arrow
question_answer 171) Venation in fern is
A)
circinate venation
done
clear
B)
reticulate venation
done
clear
C)
parallel venation
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 172)
Which of the following two characters are shared by Cycas and Pteris? Choose correct option from codes given below. I. Formation of motile gametes. II. Formation of haploid endosperm. III. Formation of sporophyte directly from gametophyte without gametic union. IV. Formation of archegonia in female gametophyte.
A)
I and IV
done
clear
B)
I and III
done
clear
C)
II and IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 173)
Arrange the following four zone in roots in chronological sequence from apex to base. I. Mineral absorption zone. II. Meristemetic zone. III. Maturation zone. IV. Water absorption zone.
A)
IV, III, II and I
done
clear
B)
II, IV, I and III
done
clear
C)
I, II, IV and III
done
clear
D)
II III, IV and I
done
clear
View Answer play_arrow
question_answer 174) Sub aerial stem modification with long internode is
A)
phyllode
done
clear
B)
phylloclade
done
clear
C)
Runner
done
clear
D)
Twinner
done
clear
View Answer play_arrow
question_answer 175) Modification of petiole into leaf like structure is called
A)
phylloclade
done
clear
B)
phyllode
done
clear
C)
pistillode
done
clear
D)
cladode
done
clear
View Answer play_arrow
question_answer 176) Spadix and hypanthodium inflorescences
A)
mulberry and banana
done
clear
B)
banana and coriander
done
clear
C)
Ficus and banana
done
clear
D)
banana and Ficus
done
clear
View Answer play_arrow
question_answer 177) In the flower of a plant, the ovarian part is fused, but style and stigma are free, its ovary becomes unilocular due to break down of partition wall and the ovules are attached to a central axis. The feature belongs to
A)
abutilon
done
clear
B)
nymphaea
done
clear
C)
dianthus
done
clear
D)
michellis
done
clear
View Answer play_arrow
question_answer 178) The condition in which gynoecium occupy topmost position of thalamus, the flower is
A)
inferior
done
clear
B)
epigynous
done
clear
C)
perigynous
done
clear
D)
hypogynous
done
clear
View Answer play_arrow
question_answer 179) Germination of seed inside fruit is a peculiar characteristic
A)
mangrooves/ halophytes
done
clear
B)
mesophytes
done
clear
C)
xerophytes
done
clear
D)
emergant hydrophytes
done
clear
View Answer play_arrow
question_answer 180) Parachute mechanism of seed and fruit dispersal is characteristic feature of family
A)
Leguminosae
done
clear
B)
Compositae
done
clear
C)
Malvaceae
done
clear
D)
Caryophyllaceae
done
clear
View Answer play_arrow
question_answer 181) Assertion The transport of water is more efficient in angiosperm because their xylem possess vessels. Reson (R) Conduction of water by vessel element is an passive process thus no energy is needed in the process.
A)
Both A and R true and R is correct explanation
done
clear
B)
Both A and R are true, but R is not correct explanation of A
done
clear
C)
A is true, but R is false
done
clear
D)
Both A and R are false
done
clear
View Answer play_arrow
question_answer 182) Which one is correct in chronological sequence regarding anatomy of roots?
A)
Cortex, epiblema, pericycle, endodermis
done
clear
B)
Cortex, epiblema, endodermis, epidermis
done
clear
C)
Epiblema, cortex, endodermis, pericycles
done
clear
D)
Epiblema, cortex, pericycle, endodermis
done
clear
View Answer play_arrow
question_answer 183) A bicollateral vascular bundle (tissue) has the following arrangement of tissues
A)
outer phloem \[\to \] outer xylem \[\to \] middle cambium inner cambium \[\to \] inner xylem\[\to \] inner phloem
done
clear
B)
outer phloem\[\to \] outer cambium \[\to \] middle xylem inner cambium \[\to \] inner phloem
done
clear
C)
outer xylem \[\to \] outer cambium \[\to \] middle phloem inner cambium \[\to \] inner xylem
done
clear
D)
outer cambium \[\to \] outer phloem \[\to \] middle xylem inner phloem \[\to \] inner cambium
done
clear
View Answer play_arrow
question_answer 184) Chronological sequence of cellular layers from the periphery towards the cortex in an old dicot stem is
A)
epidermis, hypodermis, cortex, endodermis
done
clear
B)
epidermis, phellem, phellogen, phelloderm
done
clear
C)
epidermis, hypodermis, phellogen, phelloderm
done
clear
D)
epidermis, phellogen, phellem, endodermis
done
clear
View Answer play_arrow
question_answer 185) Glyoxysomes are akin to peroxisomes. These contain specific enzymes which convert
A)
protein into peptones
done
clear
B)
fats into fatty acids
done
clear
C)
fats into sugars
done
clear
D)
glucose into \[C{{O}_{2}}\] and \[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 186) Meiosis involves two cell division, the first of which is heterotypic division, the second cell division is
A)
direct cell division
done
clear
B)
reduction cell division
done
clear
C)
homoeotypic cell division
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 187) During meiosis cell division chromosome first appear duplicated, at this stage each bivalent is bettar reffered as
A)
chromatid
done
clear
B)
tetraxon
done
clear
C)
tetrad
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 188) Transpiration rate in Sorghum is comparatively higher than maize. This is due to
A)
increased shoot/root ratio
done
clear
B)
increased rate of respiratory quotient
done
clear
C)
increased rate of photosynthesis
done
clear
D)
decreased root/shoot ratio
done
clear
View Answer play_arrow
question_answer 189) Assertion Halophytes survive in saline habitats by maintaining low internal \[N{{a}^{+}}\] levels. Reason (R) Salt resistant plant get rid off excess \[N{{a}^{+}}\] by ATP energised antiporter.
A)
Both A and R true and R is correct explanation of A
done
clear
B)
Both A and R are true, but R is not correct explanation of A
done
clear
C)
A is true, but R is false
done
clear
D)
Both A and R are false
done
clear
View Answer play_arrow
question_answer 190)
Match the words in column I with phrases in column II. Choose the correct combination from the given option. Column I Column II A. Sulphur 1. Found in plant hormone B. Zink 2. Important component for animals C. Magnesium 3. Found in amino acid like methionine D. Iodine 4. Component of sugar E. Manganese 5. Nitrogenous component F. Molybdenum 6. Structural component of photosynthetic pigment
Codes
A)
A-3 A-1 A-6 A-2 A-4 A-5
done
clear
B)
B-5 B-1 B-6 B-2 B-5 B-4
done
clear
C)
C-5 C-6 C-1 C-2 C-4 C-5
done
clear
D)
D-3 D-1 D-2 D-6 D-4 D-5
done
clear
View Answer play_arrow
question_answer 191) Which is the most efficient free nitrogen fixer cyanobacterium in field of rice?
A)
Nostoc
done
clear
B)
Tolypothrix
done
clear
C)
Aulosira
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
question_answer 192) Chlorophyll-a and chlorophyll-6 are different in having
A)
chlorophyll-a has an ethyl group and chlorophyll-b has an aldehyde group in position x.
done
clear
B)
chlorophyll-a has a carboxyl gourp and chlorophyll-b has an aldehyde group in position x
done
clear
C)
chlorophyll-a has an aldehyde group and chlorophyli-o has a methyl group in position x
done
clear
D)
chlorophyll-a has a methyl group and chlorophyll-D has aldehyde group in position x
done
clear
View Answer play_arrow
question_answer 193) Which one of the following protein/ enzyme is maximum in chloroplast?
A)
Nuclease
done
clear
B)
Phosphatase
done
clear
C)
Hexokinase
done
clear
D)
Ribulose diphosphate carboxylase
done
clear
View Answer play_arrow
question_answer 194) Respiratory quotient of oxalic acid is
A)
1.5
done
clear
B)
1.0
done
clear
C)
4
done
clear
D)
0.7
done
clear
View Answer play_arrow
question_answer 195) Cry II Ab and Cry I Ab produce toxin that control
A)
cotton bollworms and corn borer respectively
done
clear
B)
corn borer and tobacco budworms respectively
done
clear
C)
Tobacco bud worms and nematodes respectively
done
clear
D)
nematodes and tobacco budworms respectively
done
clear
View Answer play_arrow
question_answer 196) How many biosphere reserve were notified in India, under MAB programme in 1971?
A)
16
done
clear
B)
9
done
clear
C)
8
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 197) The concept of ecological pyramids were proposed by
A)
Odum
done
clear
B)
Reiter
done
clear
C)
Charles EIton
done
clear
D)
Koestler
done
clear
View Answer play_arrow
question_answer 198) The percentage of forest cover recommanded by the National Forest Policy (1988) is
A)
23% of plains and 77% for hills
done
clear
B)
77% of plains and 23% for hills
done
clear
C)
67% for hills and 33% for plains
done
clear
D)
33% for hills and 67% for plains
done
clear
View Answer play_arrow
question_answer 199) Cellulose is composed of
A)
unbranched chain of glucose molecule linked by \[\beta \]-1,4 glycosidic bond
done
clear
B)
unbranched chain of glucose molecule linked by \[\alpha \]-1.6 glycosidic bond
done
clear
C)
branched chain of glucose molecule linked by \[\alpha \]-1, 4 glycosidic bond in straight chain and \[\beta \]-1, 6 glycosidic bond at the site of branhing
done
clear
D)
branched chain of glucose molecule linked by \[\alpha \]-1, 6 glycosidic bond in straight chain and \[\beta \]-1, 4 glycosidic bond at the site of branching
done
clear
View Answer play_arrow
question_answer 200) Aconite is obtained from
A)
stem
done
clear
B)
leaves
done
clear
C)
tuberous root
done
clear
D)
dried leaves and seeds
done
clear
View Answer play_arrow