question_answer 1) An electric fan is placed on a stationary boat and air is blown with it on the sail of the boat. Which of the following statements is correct?
A)
The boat will be uniformly accelerated in the direction of the flow of the air
done
clear
B)
The boat will start moving with uniform speed
done
clear
C)
The boat will be uniformly accelerated opposite to the direction of flow of air
done
clear
D)
The boat will remain stationary as before
done
clear
View Answer play_arrow
question_answer 2) When the bob of a simple pendulum swings, the work done by tension in the string is
A)
\[>0\]
done
clear
B)
\[<0\]
done
clear
C)
zero
done
clear
D)
maximum
done
clear
View Answer play_arrow
question_answer 3) If P represents radiation pressure, c represents speed of light and Q represents radiation energy striking a unit area per second then non-zero integers\[x,\text{ }y\]and z such that\[{{P}^{x}}{{Q}^{y}}{{c}^{z}}\]is dimensionless, are
A)
\[x=1,y=1,z=-1\]
done
clear
B)
\[x=1,y=-1,z=1\]
done
clear
C)
\[x=-1,y=1,z=1\]
done
clear
D)
\[x=1,y=1,z=1\]
done
clear
View Answer play_arrow
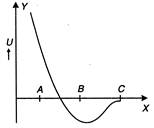
question_answer 4)
The potential energy U between two atoms in a diatomic molecule as a function of the distance\[x\]between atoms has been shown in the adjoining figure. The atoms are
A)
attracted when\[x\]lies between A and B and are repelled when\[x\]lies between B and C
done
clear
B)
attracted when\[x\]lies between B and C and are repelled when\[x\]lies between A and B
done
clear
C)
attracted when they reach B
done
clear
D)
repelled when they reach B
done
clear
View Answer play_arrow
question_answer 5) For a particle executing simple harmonic motion, the kinetic energy K is given by, \[K={{K}_{0}}{{\cos }^{2}}\omega t.\]The maximum value of potential energy is
A)
\[{{K}_{0}}\]
done
clear
B)
zero
done
clear
C)
\[{{K}_{0}}/2\]
done
clear
D)
not obtainable
done
clear
View Answer play_arrow
question_answer 6) The speed of sound in hydrogen at NTP is 1270 m/s. Then, the speed in a mixture of hydrogen and oxygen in the ratio 41 by volume will be
A)
317 m/s
done
clear
B)
635 m/s
done
clear
C)
830 m/s
done
clear
D)
950 m/s
done
clear
View Answer play_arrow
question_answer 7) A water drop is divided into 8 equal droplets. The pressure difference between the inner and outer side of the big drop will be
A)
same as for smaller droplet
done
clear
B)
\[\frac{1}{2}\]of that for smaller droplet
done
clear
C)
\[\frac{1}{4}\]of that for smaller droplet
done
clear
D)
twice that for smaller droplet
done
clear
View Answer play_arrow
question_answer 8) A train of 150 m length is going towards north direction at a speed of\[10\text{ }m{{s}^{-1}}\]. A parrot flies at a speed of\[5\text{ }m{{s}^{-1}}\]towards south direction parallel to the railway track. The time taken by the parrot to cross the train is equal to
A)
12s
done
clear
B)
8s
done
clear
C)
15s
done
clear
D)
10s
done
clear
View Answer play_arrow
question_answer 9) Two vessels A and B having equal volume contain equal masses of hydrogen in A and helium in B at 300 K. Then, mark the correct statement.
A)
The pressure exerted by hydrogen is half that exerted by helium
done
clear
B)
The pressure exerted by hydrogen is equal to that exerted by helium
done
clear
C)
Average KE of the molecule of hydrogen is half the average KE of the molecules of helium
done
clear
D)
The pressure exerted by hydrogen is twice that exerted by helium
done
clear
View Answer play_arrow
question_answer 10) A charge Q is uniformly distributed over a large square plate of copper. The electric field at a point very close to the centre of the plate is 10 V/m. If the copper plate is replaced by a plastic plate of the same geometrical dimensions and carrying the same charge Q uniformly distributed, then the electric field at the point P will be
A)
5 V/m
done
clear
B)
zero
done
clear
C)
10 V/m
done
clear
D)
20 V/m
done
clear
View Answer play_arrow
question_answer 11) A piece of wire of resistance R is cut into n equal parts. These parts are then connected in parallel. If the equivalent resistance of the parallel combination is\[R',\], then (R/R) is
A)
1/1
done
clear
B)
\[n/1\]
done
clear
C)
\[{{n}^{2}}/1\]
done
clear
D)
\[1/n\]
done
clear
View Answer play_arrow
question_answer 12) Ice starts freezing in a lake with water at\[0{}^\circ C\]when the atmospheric temperature is\[-10{}^\circ C\]. If the time taken for 1 cm of ice to be formed is 12 min; the time taken for the thickness of the ice to change from 1 cm to 2 cm will be
A)
12 min
done
clear
B)
less than 12 min
done
clear
C)
more than 12 min but less than 24 min
done
clear
D)
more than 24 min
done
clear
View Answer play_arrow
question_answer 13) An ideal gas is taken through a cyclic thermo dynamical process through four steps. The amounts of heat involved in these steps are \[{{Q}_{1}}=5960\text{ }J,\text{ }{{Q}_{2}}=-5585\text{ }J,\text{ }{{\text{Q}}_{3}}=-2980\text{ }J,\] \[{{Q}_{4}}=3645\text{ }J;\]respectively. The corresponding works involved are\[{{W}_{1}}=22000\,J,{{W}_{2}}=-825\,J,\] \[{{W}_{3}}=-1100\,J\]and\[{{W}_{4}}\]respectively. The value of\[{{W}_{4}}\]is
A)
1315 J
done
clear
B)
275 J
done
clear
C)
765J
done
clear
D)
675J
done
clear
View Answer play_arrow
question_answer 14) A beam of light consisting of red, green and blue colours is incident on a right-angled prism. The refractive indices of the material of the prism for the above red, green and blue wavelengths are 1.39, 1.44 and 1.47 respectively. The prism will
A)
separate part of the red colour from the green and blue colours
done
clear
B)
separate part of the blue colour from the red and green colours
done
clear
C)
separate all the three colours from one another
done
clear
D)
not separate even partially any colour from the other two colours
done
clear
View Answer play_arrow
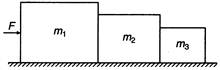
question_answer 15)
Three blocks of masses\[{{m}_{1}},{{m}_{2}}\]and\[{{m}_{3}}\]kg are placed in contact with each other on a frictionless table. A force F is applied on the heaviest mass\[{{m}_{1}};\]the acceleration of\[{{m}_{3}}\]will be
A)
\[\frac{F}{{{m}_{1}}}\]
done
clear
B)
\[\frac{F}{{{m}_{1}}+{{m}_{2}}}\]
done
clear
C)
\[\frac{F}{{{m}_{2}}+{{m}_{3}}}\]
done
clear
D)
\[\frac{F}{{{m}_{1}}+{{m}_{2}}+{{m}_{3}}}\]
done
clear
View Answer play_arrow
question_answer 16) A particle is moving along a circular path of radius 5 m with a uniform speed\[5\text{ }m{{s}^{-1}}\]. What will be the average acceleration when the particle completes half revolution?
A)
Zero
done
clear
B)
\[10\,\,m{{s}^{-2}}\]
done
clear
C)
\[10\,\pi \,m{{s}^{-2}}\]
done
clear
D)
\[\frac{10}{\pi }\,\,m{{s}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 17) ECE of copper is\[3.3\times {{10}^{-7}}kg/C\]. If 100 kWh energy is consumed at 33 V in a copper voltameter, then mass of copper liberated is
A)
3.6kg
done
clear
B)
3.3kg
done
clear
C)
1kg
done
clear
D)
\[1\,mg\]
done
clear
View Answer play_arrow
question_answer 18) A ray of light is incident on the surface of separation of a medium with the velocity of light at an angle\[45{}^\circ \]and is refracted in the medium at an angle\[30{}^\circ \]. What will be the velocity of light in the -medium?
A)
\[1.96\times {{10}^{8}}m/s\]
done
clear
B)
\[2.12\times {{10}^{8}}m/s\]
done
clear
C)
\[3.18\times {{10}^{8}}m/s\]
done
clear
D)
\[3.33\times {{10}^{8}}m/s\]
done
clear
View Answer play_arrow
question_answer 19) Two identical wires A and B have the same length L and carry the same current\[I\]. Wire A is bent into a circle of radius R and wire B is bent to form a square of side a. If\[{{B}_{1}}\]and\[{{B}_{2}}\] are the values of magnetic induction at the centre of the circle and the centre of the square respectively, then the ratio\[{{B}_{1}}/{{B}_{2}}\]is
A)
\[({{\pi }^{2}}/8)\]
done
clear
B)
\[({{\pi }^{2}}/8\sqrt{2})\]
done
clear
C)
\[({{\pi }^{2}}/16)\]
done
clear
D)
\[({{\pi }^{2}}/16\sqrt{2})\]
done
clear
View Answer play_arrow
question_answer 20) In non-resonant circuit, what will be the nature of the circuit for frequencies higher than the resonant frequency?
A)
Resistive
done
clear
B)
Capacitive
done
clear
C)
Inductive
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 21) A particle of mass m is moving in a circular path of constant radius r such that centripetal acceleration\[{{a}_{c}}\]varying with time is\[{{a}_{c}}={{k}^{2}}r{{t}^{2}}\]where k is a constant. What is the power delivered to the particle by the force acting on it?
A)
\[2mk{{r}^{2}}t\]
done
clear
B)
\[mk{{r}^{2}}{{t}^{2}}\]
done
clear
C)
\[m{{k}^{2}}{{r}^{2}}t\]
done
clear
D)
\[m{{k}^{2}}r{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 22) The maximum height attained by a projectile is increased by 5%. Keeping the angle of projection constant, what is the percentage increase in horizontal range?
A)
5%
done
clear
B)
10%
done
clear
C)
15%
done
clear
D)
20%
done
clear
View Answer play_arrow
question_answer 23) A spot of light S rotates in a horizontal plane with a constant angular velocity of 0.1 rad/s. The spot of light P moves along the wall at a distance of 3 m from S. The velocity of spot P, where\[\theta =45{}^\circ ,\]is
A)
0.5 m/s
done
clear
B)
0.6 m/s
done
clear
C)
0.7 m/s
done
clear
D)
0.8 m/s
done
clear
View Answer play_arrow
question_answer 24) White light may be considered to be a mixture of wave with\[\lambda \]ranging between \[3000\overset{\text{o}}{\mathop{\text{A}}}\,\] and \[7800\overset{\text{o}}{\mathop{\text{A}}}\,\]. An oil film of thickness \[10000\overset{\text{o}}{\mathop{\text{A}}}\,\] is examined normally by the reflected light. If \[\mu =1.4,\]then the film appears bright for
A)
\[4308\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }5091\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }6222\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4000\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }5091\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ 5600}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4667\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ 6222}\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ 7000}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[4000\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ 4667}\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ 5600}\overset{\text{o}}{\mathop{\text{A}}}\,,\,\,70\text{00}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 25) To get the maximum flight a ball must be thrown as
A)
done
clear
B)
done
clear
C)
done
clear
D)
any of , and
done
clear
View Answer play_arrow
question_answer 26) Rain is falling vertically downwards with a velocity of 4 km/h. A man walks in the rain with a velocity of 3 km/h. The raindrops will fall on the man with a velocity of
A)
1 km/h
done
clear
B)
3 km/h
done
clear
C)
4 km/h
done
clear
D)
5 km/h
done
clear
View Answer play_arrow
question_answer 27) A machine gun is mounted on a 200 kg vehicle on a horizontal smooth road (friction negligible). The gun fires 10 bullets per sec with a velocity of 500 m/s. If the mass of each bullet be 10 g, what is the acceleration produced in the vehicle?
A)
\[25cm/{{s}^{2}}\]
done
clear
B)
\[25\text{ r}r/{{s}^{2}}\]
done
clear
C)
\[50cm/{{s}^{2}}\]
done
clear
D)
\[50\,rr/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 28) The displacement of a particle executing periodic motion is given by \[y=4{{\cos }^{2}}(t/2)\sin (1000t)\] This expression may be considered to be a result of superposition of
A)
two waves
done
clear
B)
three waves
done
clear
C)
four waves
done
clear
D)
five waves
done
clear
View Answer play_arrow
question_answer 29) If the series limit wavelength of the Lyman series for hydrogen atom is \[912\overset{\text{o}}{\mathop{\text{A}}}\,\] then the series limit wavelength for the Balmer series for the hydrogen atom is
A)
\[912\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[912\times 2\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[912\times 4\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[\frac{912}{2}\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 30) Assuming that about 20 MeV of energy is released per fusion reaction \[_{1}{{H}^{2}}{{+}_{1}}{{H}^{3}}{{\to }_{0}}{{n}^{1}}{{+}_{2}}H{{e}^{4}}\] then the mass of\[_{1}{{H}^{2}}\]consumed per day in a fusion reactor of power 1 MW will approximately be
A)
0.001 g
done
clear
B)
0.1 g
done
clear
C)
10.0 g
done
clear
D)
1000 g
done
clear
View Answer play_arrow
question_answer 31) A pure semiconductor behaves slightly as a conductor at
A)
room temperature
done
clear
B)
low temperature
done
clear
C)
high temperature
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 32) A coil has an inductance of 0.7 H and is joined in series with a resistance of\[220\,\Omega \]. When an alternating emf of 220 V at 50 cps is applied to it, then the wattles component of the current in the circuit is
A)
5 A
done
clear
B)
0.5 A
done
clear
C)
0.7 A
done
clear
D)
7 A
done
clear
View Answer play_arrow
question_answer 33) Which of the following statements is /are true?
A)
A clock when taken on a mountain can be made to give correct time if we change the length of pendulum suitably
done
clear
B)
An increase in value of g makes a clock go slow
done
clear
C)
If the length of a pendulum is increased, the clock becomes fast
done
clear
D)
A clock when taken to a deep mine or carried to the top of a mountain becomes slow
done
clear
View Answer play_arrow
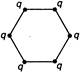
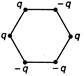
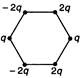
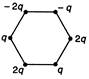
question_answer 34) Figure below show regular hexagons?, with charges at the vertices. In which case is the electric field at the centre zero?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 35) There is a current of 40 A in a wire of\[{{10}^{-6}}{{m}^{2}}\]area of cross-section. If the number of free electrons per cubic metre is\[{{10}^{29}},\]then the drift velocity is
A)
\[250\times {{10}^{-3}}m/s\]
done
clear
B)
\[25.0\times {{10}^{-3}}m/s\]
done
clear
C)
\[2.50\times {{10}^{-3}}m/s\]
done
clear
D)
\[1.25\times {{10}^{3}}m/s\]
done
clear
View Answer play_arrow
question_answer 36) A mixture of hi moles of monoatomic gas and moles of diatomic gas has\[\frac{{{C}_{p}}}{{{C}_{V}}}=\gamma =1.5\]. Then
A)
\[{{n}_{1}}={{n}_{2}}\]
done
clear
B)
\[2{{n}_{1}}={{n}_{2}}\]
done
clear
C)
\[{{n}_{1}}=2{{n}_{2}}\]
done
clear
D)
\[2{{n}_{1}}=3{{n}_{2}}\]
done
clear
View Answer play_arrow
question_answer 37) Change in frequency due to Dopple?s effect is produced when
A)
the source and the observer are moving in the same direction
done
clear
B)
the source and the observer are both at rest
done
clear
C)
there is a relative motion between the source and the observer
done
clear
D)
there is a resultant motion between the source and observer
done
clear
View Answer play_arrow
question_answer 38) A dip needle arranged to move freely in the magnetic meridian dips by an angle\[\theta \]. If the vertical plane in which the needle moves is rotated through an angle a to the magnetic meridian, then the needle will dip by an angle
A)
\[\theta \]
done
clear
B)
\[\alpha \]
done
clear
C)
more than\[\theta \]
done
clear
D)
less than\[\theta \]
done
clear
View Answer play_arrow
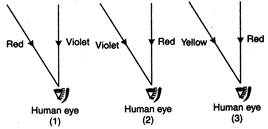
question_answer 39)
The coming rays are forming rainbows
A)
Figure (1) forms primary rainbow
done
clear
B)
Figure (1) and (3) form primary rainbow
done
clear
C)
Figure (2) forms secondary rainbow
done
clear
D)
Figure (1) forms secondary rainbow
done
clear
View Answer play_arrow
question_answer 40) A table tennis ball which has been covered with a conducting paint is suspended by a silk thread so that it hangs between two metal plates. One plate is earthed. When the other. plate is connected to a high voltage generator, the ball
A)
is attracted to the high voltage plate and stays there
done
clear
B)
hangs without moving
done
clear
C)
swings backward and forward hitting each plate in turn
done
clear
D)
is repelled to the earthed plate and stays there
done
clear
View Answer play_arrow
question_answer 41) In a step-up transformer, the turn ratio is 1 2 . A Leclanche cell (emf = 1.5 V) is connected across the primary. The voltage developed in the secondary would be
A)
3.0V
done
clear
B)
0.75V
done
clear
C)
1.5V
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 42) If the total magnetic field due to earth is 28 A/m, then the total magnetic induction due to earth is
A)
28 T
done
clear
B)
280 ab-amp/cm
done
clear
C)
0.352 gauss
done
clear
D)
0.352 T
done
clear
View Answer play_arrow
question_answer 43) In an adiabatic process wherein pressure is increased by\[\frac{2}{3}%\]. If\[\frac{{{C}_{p}}}{{{C}_{V}}}=\frac{3}{2},\]then the volume decreases by about
A)
\[\frac{4}{9}%\]
done
clear
B)
\[\frac{2}{3}%\]
done
clear
C)
\[4%\]
done
clear
D)
\[\frac{9}{4}%\]
done
clear
View Answer play_arrow
question_answer 44) The wavelength of the\[{{K}_{\alpha }}\]line for an element of atomic number 43 is\[\lambda .\]Then the wavelength of the\[{{K}_{\alpha }}\]line for an element of atomic number
A)
\[\left( \frac{43}{29} \right)\lambda \]
done
clear
B)
\[\left( \frac{42}{28} \right)\lambda \]
done
clear
C)
\[\left( \frac{9}{4} \right)\lambda \]
done
clear
D)
\[\left( \frac{4}{9} \right)\lambda \]
done
clear
View Answer play_arrow
question_answer 45) The process of adding impurities to the pure semiconductor is called
A)
drouping
done
clear
B)
drooping
done
clear
C)
doping
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 46) The radioactivity of a sample is\[{{R}_{1}}\]at a time\[{{T}_{1}}\]and\[{{R}_{2}}\]at times\[{{T}_{2}}\]. the half-life of the specimen is T, the number of atoms that have disintegrated at the time\[({{T}_{2}}-{{T}_{1}})\]is proportional to
A)
\[{{R}_{1}}{{T}_{1}}-{{R}_{2}}{{T}_{2}}\]
done
clear
B)
\[{{R}_{1}}-{{R}_{2}}\]
done
clear
C)
\[\frac{{{R}_{1}}-{{R}_{2}}}{T}\]
done
clear
D)
\[({{R}_{1}}-{{R}_{2}})T\]
done
clear
View Answer play_arrow
question_answer 47) A proton, a deuteron and an\[\alpha -\]particle with the same KE enter a region of uniform magnetic field, moving at right angles to B. What is the ratio of the radii of their circular paths?
A)
\[1:\sqrt{2}:1\]
done
clear
B)
\[1:\sqrt{2}:\sqrt{2}\]
done
clear
C)
\[\sqrt{2}:1:1\]
done
clear
D)
\[\sqrt{2}:\sqrt{2}:1\]
done
clear
View Answer play_arrow
question_answer 48) Two streams of protons move parallel to each other in the same direction. Then these
A)
do not interact at all
done
clear
B)
attract each other
done
clear
C)
repel each other
done
clear
D)
get rotated to be 1 to each other
done
clear
View Answer play_arrow
question_answer 49) The ratio of the speed of an object to the speed of its real image of magnification m of a convex mirror is
A)
\[-1/{{m}^{2}}\]
done
clear
B)
\[{{m}^{2}}\]
done
clear
C)
\[-m\]
done
clear
D)
\[1/m\]
done
clear
View Answer play_arrow
question_answer 50) The maximum current that flows in the fuse wire, before it blows out, varies with the radius r as
A)
\[{{r}^{3/2}}\]
done
clear
B)
r
done
clear
C)
\[{{r}^{2/3}}\]
done
clear
D)
\[{{r}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 51) The incorrect statement is
A)
circumference of electron orbit\[(2\pi r)=n\lambda \] (n = an integer)
done
clear
B)
de-Broglie equation:\[\lambda =\frac{n}{mv}\]
done
clear
C)
angular momentum of an electron\[\left( \frac{h}{mv}=\frac{2\pi r}{n}or\,mvr=n\frac{h}{2\pi } \right)\]is an integral multiple of\[\frac{h}{2\pi }\]
done
clear
D)
angular momentum of the electron is not quantized
done
clear
View Answer play_arrow
question_answer 52) Which of the following is not possible for\[4p\]or\[3d\]electrons?
A)
\[n=3,l=2,m=+1,s=+\frac{1}{2}\]
done
clear
B)
\[n=4,l=1,m=0,s=+\frac{1}{2}\]
done
clear
C)
\[n=3,l=3,m=+3,s=+\frac{1}{2}\]
done
clear
D)
\[n=4,l=1,m=-1,s=+\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 53) Assertion :\[H{{e}_{2}}\]does not exist. Reason (R): Bond-order of\[H{{e}_{2}}\]is zero.
A)
A and R both are correct and R is me correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A is incorrect but R is correct.
done
clear
D)
Both A and R are incorrect.
done
clear
View Answer play_arrow
question_answer 54) Internal energy for one mole of an ideal gas is
A)
\[\frac{3}{2}RT\]
done
clear
B)
\[\frac{3}{2}kT\]
done
clear
C)
\[\frac{1}{2}RT\]
done
clear
D)
\[\frac{1}{2}kT\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following is correct?
A)
\[{{C}_{V}}={{\left( \frac{\delta u}{\delta T} \right)}_{p}}\]
done
clear
B)
\[{{\left( \frac{\delta H}{\delta T} \right)}_{V}}\]
done
clear
C)
\[{{C}_{p}}-{{C}_{V}}=R\] for one mole of an ideal gas
done
clear
D)
\[{{\left( \frac{\delta u}{\delta V} \right)}_{T}}=-\frac{a}{{{V}^{2}}}\](internal pressure in van der Waals' equation)
done
clear
View Answer play_arrow
question_answer 56) The correct order of decreasing dipole moment of (I) toluene, (II) m -dichlorobenzene , (III) o-dichlorobenzene and (IV) p -dichlorobenzene is
A)
IV < II < I < III
done
clear
B)
IV < I < II < III
done
clear
C)
I < IV < II < III
done
clear
D)
IV < I < III < II
done
clear
View Answer play_arrow
question_answer 57) Which of the following is weakest?
A)
Ionic
done
clear
B)
Covalent
done
clear
C)
van der Waals forces
done
clear
D)
Metallic bond
done
clear
View Answer play_arrow
question_answer 58) The reaction favoured at low pressure is
A)
\[{{H}_{2}}(g)+{{I}_{2}}(g)2HI(g)\]
done
clear
B)
\[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
C)
\[{{N}_{2}}(g)+{{O}_{2}}(g)2NO(g)\]
done
clear
D)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)\]
done
clear
View Answer play_arrow
question_answer 59) According to Bronsted, a base can
A)
accept protons
done
clear
B)
donate protons
done
clear
C)
lose a pair of electrons
done
clear
D)
gain a pair of electrons
done
clear
View Answer play_arrow
question_answer 60) Which of the following is incorrect?
A)
\[{{H}_{2}}{{O}_{2}}\]is a weak acid
done
clear
B)
\[{{H}_{2}}{{O}_{2}}\]is a weak alkali
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\]acts as an oxidising agent
done
clear
D)
\[{{H}_{2}}{{O}_{2}}\]is a reducing agent
done
clear
View Answer play_arrow
question_answer 61) Diagonal relationship is not shown by
A)
Li and Mg
done
clear
B)
Be and Al
done
clear
C)
B and Si
done
clear
D)
C and P
done
clear
View Answer play_arrow
question_answer 62) The IUPAC name of\[{{(C{{H}_{3}})}_{2}}CHC{{H}_{3}}\]is
A)
dimethylethane
done
clear
B)
trimethylmethane
done
clear
C)
isopropylmethane
done
clear
D)
2-methyl propane
done
clear
View Answer play_arrow
question_answer 63) Which of the following is incorrect?
A)
Functional isomerism is shown by \[C{{H}_{3}}C{{H}_{2}}OH\text{ }and\text{ }C{{H}_{3}}OC{{H}_{3}}\]
done
clear
B)
\[H-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-H\xrightarrow{Conc.\,{{H}_{2}}S{{O}_{4}}}\]\[H-\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}\,=\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}\,-H+{{H}_{2}}O\]
done
clear
C)
Glucose is a monosaccharide sugar
done
clear
D)
Fructose is a disaccharide sugar
done
clear
View Answer play_arrow
question_answer 64) The strongest acid is
A)
\[CH\equiv CH\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
D)
\[C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 65) Assertion : Graphite is soft while diamond is very hard. Reason (R): Graphite has three dimensional structure while diamond has planar.
A)
A and R both are correct and R is the correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A is correct but R is incorrect.
done
clear
D)
A is incorrect while R is correct.
done
clear
View Answer play_arrow
question_answer 66) Which of the following is incorrect?
A)
A real gas behaves like an ideal gas over a wide range of pressure(\[\sim 100\]atm) at Boyle point.
done
clear
B)
A real gas behaves like an ideal gas over a wide range of pressure (\[\sim 100\]atm) at critical temperature of the gas.
done
clear
C)
\[{{\left( \frac{\delta u}{\delta V} \right)}_{T}}=0\]for an ideal gas.
done
clear
D)
\[{{\left( \frac{\delta u}{\delta V} \right)}_{T}}=\frac{a}{{{V}^{2}}}\]for a real gas obeying van der Waals' equation.
done
clear
View Answer play_arrow
question_answer 67) The homogeneous catalysis is shown by
A)
Haber?s process: \[{{N}_{2}}(g)+3{{H}_{2}}(g)\xrightarrow[{}]{Fe(s)}2N{{H}_{3}}(g)\]
done
clear
B)
Ostwald process: \[4N{{H}_{3}}(g)+5{{O}_{2}}(g)\xrightarrow[{}]{Pt(s)}\]
done
clear
C)
Contact process: \[2S{{O}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{Pt(s)}2S{{O}_{3}}(g)\]
done
clear
D)
\[2{{C}_{2}}{{H}_{5}}OH(aq)\xrightarrow{{{H}_{2}}S{{O}_{4}}(aq)}\] \[{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}(aq)+{{H}_{2}}O(l)\]
done
clear
View Answer play_arrow
question_answer 68) Which of the following is incorrect?
A)
Invertase carries the hydrolysis of sugar into glucose and fructose.
done
clear
B)
Starch is converted into maltose with the help of diastase.
done
clear
C)
Maltase helps in converting maltose into glucose.
done
clear
D)
Maltase helps in converting starch into maltose.
done
clear
View Answer play_arrow
question_answer 69) Which of the following is incorrect match?
A)
done
clear
B)
Name of colloidal solution Examples Dispersed phase Dispersion medium Foam Soap leather, soda water Gas Solid
done
clear
C)
Name of colloidal solution Examples Dispersed phase Dispersion medium Solid foam Rubber, bread Gas Solid
done
clear
D)
Name of colloidal solution Examples Dispersed phase Dispersion medium Aerosol Mist, for, cloud Liquid Solid
Name of colloidal solution Examples Dispersed phase Dispersion medium Emulsion Milk, cod liver oil Liquid Liquid
done
clear
View Answer play_arrow
question_answer 70) Assertion : The ionic size of\[M{{g}^{2+}}>A{{l}^{3+}}\] Reason (R): In isoelectronic species, greater the nuclear charge, less is the size.
A)
A and R both are correct and R is the correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A is incorrect while R is correct.
done
clear
D)
A and R both are incorrect.
done
clear
View Answer play_arrow
question_answer 71) Assertion : Silica is soluble in HF. Reason (R): \[Si{{O}_{2}}+4HF\xrightarrow[{}]{{}}Si{{F}_{4}}+2{{H}_{2}}O\] \[Si{{F}_{4}}+2HF\xrightarrow[{}]{{}}{{H}_{2}}Si{{F}_{6}}\]
A)
A and R both are correct and R is the correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A and R both are incorrect.
done
clear
D)
A is correct while R is incorrect.
done
clear
View Answer play_arrow
question_answer 72) Total number of rare earth elements is
A)
8
done
clear
B)
32
done
clear
C)
14
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 73) In the reduction of\[KMn{{O}_{4}}\]by warm acidified oxalic acid, the oxidation number of Mn changes from
A)
+ 4 to + 2
done
clear
B)
+ 6 to + 4
done
clear
C)
+ 7 to +2
done
clear
D)
+ 7 to + 4
done
clear
View Answer play_arrow
question_answer 74) Assertion :\[{{[Co{{F}_{6}}]}^{3-}}\]is paramagnetic. Reason (R):\[C{{o}^{3+}}\]has\[3{{d}^{6}}\]outer electronic configuration. The unpaired electrons do not pair up because of weak field provided by\[{{F}^{-}}\].
A)
A and R both are correct and R is the correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A is incorrect while R is correct.
done
clear
D)
A and R both are incorrect.
done
clear
View Answer play_arrow
question_answer 75) In the nuclear reaction:\[_{4}^{9}Be+_{1}^{1}H\to X+_{2}^{4}He,\] the X is
A)
\[_{2}^{4}He\]
done
clear
B)
\[_{3}^{6}Li\]
done
clear
C)
\[_{3}^{7}Li\]
done
clear
D)
\[_{4}^{8}Be\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following has the highest electron releasing tendency?
A)
\[{{F}^{-}}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[NH_{2}^{-}\]
done
clear
D)
\[CH_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following is incorrect?
A)
Primary alcohols are easily oxidised to aldehydes, which are oxidised to acids with the same number of C-atoms.
done
clear
B)
Secondary alcohols are easily oxidised to ketones, which are oxidised to acids with the same number of C-atoms.
done
clear
C)
Secondary alcohols are easily oxidised to ketones, which are oxidised to acids with lesser number of C-atoms.
done
clear
D)
Secondary and tertiary alcohols on oxidation form acids with lesser number of C-atoms.
done
clear
View Answer play_arrow
question_answer 78) For the reaction: \[{{C}_{2}}{{H}_{5}}OH+HX\xrightarrow[(anhyd.)]{ZnC{{l}_{2}}}{{C}_{2}}{{H}_{5}}X+{{H}_{2}}O\] The order of reactivity of HX is shown by
A)
\[HBr>HCl>HI\]
done
clear
B)
\[HI>HCl>HBr\]
done
clear
C)
\[HI>HBr>HCl\]
done
clear
D)
\[HCl>HBr>HI\]
done
clear
View Answer play_arrow
question_answer 79) The compound which cannot reduce Fehling solution is
A)
\[HCHO\]
done
clear
B)
\[HCOOH\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 80) The correct order of basic strengths in benzene solution is
A)
\[C{{H}_{3}}N{{H}_{2}}>{{(C{{H}_{3}})}_{2}}NH>{{(C{{H}_{3}})}_{3}}N\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}NH>{{(C{{H}_{3}})}_{3}}N>C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}N>C{{H}_{3}}N{{H}_{2}}>{{(CH{{ }_{3}})}_{2}}NH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}N>{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 81) Which of the following will not give primary amine?
A)
\[C{{H}_{3}}CON{{H}_{2}}\xrightarrow[{}]{B{{r}_{2}}/KOH}\]
done
clear
B)
\[C{{H}_{3}}CN\xrightarrow[{}]{LiAl{{H}_{4}}}\]
done
clear
C)
\[C{{H}_{3}}NC\xrightarrow[{}]{LiAl{{H}_{4}}}\]
done
clear
D)
\[C{{H}_{3}}CON{{H}_{2}}\xrightarrow[{}]{LiAl{{H}_{4}}}\]
done
clear
View Answer play_arrow
question_answer 82) Bakelite is obtained from phenol by reacting it with
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
acetal
done
clear
C)
\[HCHO\]
done
clear
D)
chlorobenzene
done
clear
View Answer play_arrow
question_answer 83) Nylon- 66 is
A)
polyamide
done
clear
B)
polyester
done
clear
C)
polystyrene
done
clear
D)
polyvinyl
done
clear
View Answer play_arrow
question_answer 84) Vitamin\[{{B}_{12}}\]contains
A)
Fe (II)
done
clear
B)
Co (III)
done
clear
C)
Zn (II)
done
clear
D)
Ca (II)
done
clear
View Answer play_arrow
question_answer 85) Which of the following is not true for antibiotics?
A)
Tetracycline is one of the broad spectrum antibiotics which are effective against a large number of harmful micro-organisms.
done
clear
B)
Streptomycin is highly effective against micro-organisms which cause tuberculosis.
done
clear
C)
Penicillin has a narrow spectrum and certain persons are sensitive to it.
done
clear
D)
Penicillin may be administered without testing the patients for sensitivity to it.
done
clear
View Answer play_arrow
question_answer 86) Assertion : Rate of reaction increases with increase in temperature. Reason (R): Number of collision increases with increase in temperature.
A)
A and R both are correct and R is the correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A is correct but R is incorrect.
done
clear
D)
A and R both are incorrect.
done
clear
View Answer play_arrow
question_answer 87) Which of the following is incorrect match for Hybridisation and geometry?
A)
Hybridistaion Geometry \[ds{{p}^{2}}\] Planar
done
clear
B)
\[{{d}^{3}}s\]and\[s{{p}^{3}}\] Tetragedrak
done
clear
C)
\[{{d}^{2}}s{{p}^{3}}\]and\[s{{p}^{3}}{{d}^{2}}\] Octahedral
done
clear
D)
\[{{d}^{3}}s\] Planar
done
clear
View Answer play_arrow
question_answer 88) Assertion : Meniscus of a liquid disappears at the critical temperature. Reason (R): Density of liquid and its gaseous phase become equal at the critical temperature.
A)
A and R both are correct and R is the correct explanation of A.
done
clear
B)
A and R both are correct but R is not the correct explanation of A.
done
clear
C)
A is correct but R is incorrect.
done
clear
D)
A is incorrect while R is correct.
done
clear
View Answer play_arrow
question_answer 89) Which of the following is correct?
A)
The outer electronic configuration of Cu (29) and Cr (24) are\[3{{d}^{9}},4{{s}^{2}}\]and\[3{{d}^{4}},4{{s}^{2}}\] respectively.
done
clear
B)
\[C{{H}_{3}}CHO\]gives hexamethylenetetramine with\[N{{H}_{3}}\]
done
clear
C)
\[\Delta G=\Delta G{}^\circ \]at equilibrium
done
clear
D)
\[{{A}_{2}}(g)+{{B}_{2}}(g)2AB(g)\]is unaffected by variations in pressure.
done
clear
View Answer play_arrow
question_answer 90) Which of the following is not true?
A)
In a first order reaction, the half-life is independent of the initial concentration of reactant.
done
clear
B)
A given piece of charcoal shows increase in its surface area in its powdered form.
done
clear
C)
In valence bond of\[{{H}_{2}},\]each electron spends its time around its own nucleus.
done
clear
D)
In valence bond of\[{{H}_{2}},\]both the electrons spend their time around both the nucleus.
done
clear
View Answer play_arrow
question_answer 91) The ground state electronic configuration of nitrogen is represented by
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 92) Which of the following sets of quantum numbers belong to highest energy?
A)
\[n=4,\text{ }l=0,\text{ }m=0\text{ }and\text{ s}=+\frac{1}{2}\]
done
clear
B)
\[n=3,\text{ }l=0,\text{ }m=0\text{ }and\text{ }s=+\frac{1}{2}\]
done
clear
C)
\[n=3,\text{ }l=1,\text{ }m=+1\text{ }and\text{ }s=+\frac{1}{2}\]
done
clear
D)
\[n=3,\text{ }l=2,\text{ }m=+1\text{ }and\text{ }s=+\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 93) The correct order of increasing energy of atomic orbitals is
A)
\[5p<4f<6s<5d\]
done
clear
B)
\[5p<65<4p<5d\]
done
clear
C)
\[4f<5p<5d<6s\]
done
clear
D)
\[5p<5d<4f<6s\]
done
clear
View Answer play_arrow
question_answer 94) In a solid lattice, the cation has left a lattice site and is located at an interstitial position. The lattice defect is
A)
interstitial defect
done
clear
B)
vacancy defect
done
clear
C)
Frenkel defect
done
clear
D)
Schottky defect
done
clear
View Answer play_arrow
question_answer 95) Schottky defect in crystals is observed when.
A)
unequal number of cations and anions is missing from the crystal lattice.
done
clear
B)
equal number of cations and anions is missing from the crystal lattice.
done
clear
C)
an anion leaves its normal site and occupies an interstitial site.
done
clear
D)
density of the crystal is increased.
done
clear
View Answer play_arrow
question_answer 96) Which of the following represents the molarity of pure water?
A)
55.5
done
clear
B)
56.5
done
clear
C)
50.5
done
clear
D)
57.55
done
clear
View Answer play_arrow
question_answer 97) The set of quantum numbers not applicable for an electron in an atoms is
A)
\[n=1,l=1,{{m}_{l}}=1,{{m}_{s}}=+\frac{1}{2}\]
done
clear
B)
\[n=1,l=0,{{m}_{l}}=0,{{m}_{s}}=+\frac{1}{2}\]
done
clear
C)
\[n=2,l=1,{{m}_{l}}=1,{{m}_{s}}=-\frac{1}{2}\]
done
clear
D)
\[n=2,l=0,{{m}_{l}}=0,{{m}_{s}}=+\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 98) The effect of temperature on reaction rate is shown by
A)
Kirchoff?s equation
done
clear
B)
Arrhenius equation
done
clear
C)
Gibb's-Helmholtz equation
done
clear
D)
Clasius-Clapeyron equation
done
clear
View Answer play_arrow
question_answer 99) Identify the incorrect statement among the following
A)
d-block elements show irregular and erratic chemical properties among themselves
done
clear
B)
La and Lu have partially filled d orbitals and no other partially filled orbitals
done
clear
C)
The chemistry of various lanthanoids is very similar
done
clear
D)
\[4f\]and\[5f\]orbitals are equally shielded
done
clear
View Answer play_arrow
question_answer 100) Which one of the following has a square planar geometry?
A)
\[{{[CoC{{l}_{4}}]}^{2-}}\]
done
clear
B)
\[{{[FeC{{l}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[NiC{{l}_{4}}]}^{2-}}\]
done
clear
D)
\[{{[PtC{{l}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 101) Rabbit is
A)
carnivore
done
clear
B)
herbivore
done
clear
C)
Both and
done
clear
D)
sanguirore
done
clear
View Answer play_arrow
question_answer 102) Medusa is the reproductive organ of the organism
A)
Hydra
done
clear
B)
Aurelia
done
clear
C)
Obelia
done
clear
D)
Sea anemone
done
clear
View Answer play_arrow
question_answer 103) Which of the following organs is the blood bank?
A)
Heart
done
clear
B)
Lungs
done
clear
C)
Spleen
done
clear
D)
Liver
done
clear
View Answer play_arrow
question_answer 104) Cavity of coelenterates is called
A)
coelenteron
done
clear
B)
coelom
done
clear
C)
cavity
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 105) Allergy involves
A)
\[\text{IgE}\]
done
clear
B)
\[\text{IgF}\]
done
clear
C)
\[\text{IgA}\]
done
clear
D)
\[\text{IgM}\]
done
clear
View Answer play_arrow
question_answer 106) Frog's tadpode is
A)
uricotelic
done
clear
B)
ureotelic
done
clear
C)
ammonotelic
done
clear
D)
aminotelic
done
clear
View Answer play_arrow
question_answer 107) RBCs are nucleated in
A)
man
done
clear
B)
rabbit
done
clear
C)
frog
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 108) 2n -1 condition is called
A)
trisomy
done
clear
B)
monosomy
done
clear
C)
nullisomy
done
clear
D)
tetrasomy
done
clear
View Answer play_arrow
question_answer 109) Giant chromosomes are found inside
A)
nucleus of man
done
clear
B)
oocyres of frog
done
clear
C)
salivary glands of silk moth
done
clear
D)
salivary glands of Drosophila
done
clear
View Answer play_arrow
question_answer 110) All Bowman's capsules of the kidney are found in
A)
pelvis
done
clear
B)
medulla
done
clear
C)
cortex
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 111) Which of the following is the most primitive ancestor of man?
A)
Homo neanderthalensis
done
clear
B)
Homo habilis
done
clear
C)
Ramapithecus
done
clear
D)
Auscralopithecus
done
clear
View Answer play_arrow
question_answer 112) Rhabditi form is the larva of
A)
Hydra
done
clear
B)
Plaryhelmin the
done
clear
C)
Ascaris
done
clear
D)
Earthworm
done
clear
View Answer play_arrow
question_answer 113) Sex determination ratio in an organism is given\[\frac{X}{A}=1.5,\] then organism will be
A)
male
done
clear
B)
female
done
clear
C)
super female
done
clear
D)
intersex
done
clear
View Answer play_arrow
question_answer 114) Hepatitis-B is also called
A)
epidemic jaundice
done
clear
B)
serum jaundice
done
clear
C)
catarrhal jaundice
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 115) Hormone that promotes cell division, protein synthesis and bone growth is
A)
ADH
done
clear
B)
ACTH
done
clear
C)
PTH
done
clear
D)
GH
done
clear
View Answer play_arrow
question_answer 116) Persons with Klinefelter syndrome have chromosomes
A)
\[\text{XX}\]
done
clear
B)
\[\text{XY}\]
done
clear
C)
\[\text{XXY}\]
done
clear
D)
\[\text{XYY}\]
done
clear
View Answer play_arrow
question_answer 117) Theory of pangenesis was given by
A)
Darwin
done
clear
B)
Lamarck
done
clear
C)
Hugo de Vries
done
clear
D)
Oparin
done
clear
View Answer play_arrow
question_answer 118) Which of the following pairs is correct
A)
E coli?Entamoeba histolytica
done
clear
B)
Culex?Elephantiasis
done
clear
C)
Bed bug?Kala-azar
done
clear
D)
Plasmodium?Sleeping sickness
done
clear
View Answer play_arrow
question_answer 119) Organisms living in open sea are called
A)
planktons
done
clear
B)
nektons
done
clear
C)
pelagic
done
clear
D)
benthos
done
clear
View Answer play_arrow
question_answer 120) Bacterium, which is concerned with pertussis is
A)
Bordetella pertussis
done
clear
B)
Bacillus
done
clear
C)
Diplococcus
done
clear
D)
Mycobacterium tuberculum
done
clear
View Answer play_arrow
question_answer 121) Different colours of frog's skin are controlled by
A)
hormones
done
clear
B)
melanocytes
done
clear
C)
nervous system
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 122) Blastula of frog has
A)
blastopore
done
clear
B)
blastocoel
done
clear
C)
archenteron
done
clear
D)
gastropore
done
clear
View Answer play_arrow
question_answer 123) Carotene pigment is found in the cells of
A)
dermis
done
clear
B)
epidermis
done
clear
C)
adipose cell
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 124) Debove's membrane is a layer of
A)
muscular tissue
done
clear
B)
epithelial tissue
done
clear
C)
connective tissue
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 125) Achilles tendon is associated with
A)
gluteus muscle
done
clear
B)
hamstring muscle
done
clear
C)
quadriceps muscle
done
clear
D)
gastrocnemius muscle
done
clear
View Answer play_arrow
question_answer 126) In the life cycle of mosquito, comma-shaped stage is
A)
larval stage
done
clear
B)
pupal stage
done
clear
C)
imago stage
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 127) Haemocoel is found in
A)
Hydra and Aurelia
done
clear
B)
Taenia and Ascaris
done
clear
C)
Cockroach and Pila
done
clear
D)
Balanoglossus and Herdmania
done
clear
View Answer play_arrow
question_answer 128) The group of an amniota includes
A)
reptiles and birds
done
clear
B)
birds and mammals
done
clear
C)
fishes and amphibians
done
clear
D)
reptiles and mammals
done
clear
View Answer play_arrow
question_answer 129) The excretory material of bony fish is
A)
urea
done
clear
B)
protein
done
clear
C)
ammonia
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 130) Which of the following variations are temporary and have nothing to do with the or next generation?
A)
Hereditary variations
done
clear
B)
Discontinuous variations
done
clear
C)
Environmental variations
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 131) The modem man differs from the apes in
A)
protruding eyes
done
clear
B)
spare body hair
done
clear
C)
wearing of clothes
done
clear
D)
arms shorter than legs
done
clear
View Answer play_arrow
question_answer 132) The leucocytes contain which of the following in large quantity ?
A)
Basophils
done
clear
B)
Neutrophils
done
clear
C)
Eosinophils
done
clear
D)
Monocytes
done
clear
View Answer play_arrow
question_answer 133) Which part of our body secretes the hormone secretin ?
A)
Ileum
done
clear
B)
Stomach
done
clear
C)
Duodenum
done
clear
D)
Oesophagus
done
clear
View Answer play_arrow
question_answer 134) During inspiration, the diaphragm
A)
expands
done
clear
B)
shows no change
done
clear
C)
contracts and flattens
done
clear
D)
relaxes to become dome-shaped
done
clear
View Answer play_arrow
question_answer 135) The oxygen toxicity is related with
A)
blood poisoning
done
clear
B)
collapsing of alveolar walls
done
clear
C)
failure of ventilation of lungs
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 136) Cardiac output is determined by
A)
heart rate
done
clear
B)
stroke volume
done
clear
C)
blood How
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 137) The important function of lymph is to
A)
transport oxygen to the brain
done
clear
B)
transport carbon dioxide to the lungs
done
clear
C)
return RBCs to the lymph nodes
done
clear
D)
return in terstial fluid to the blood
done
clear
View Answer play_arrow
question_answer 138) The lining of intestine and kidneys in human is
A)
keratinized
done
clear
B)
brush bordered
done
clear
C)
ciliated
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 139) Which of the following is not vestigial in man?
A)
Tail vertebrae
done
clear
B)
Nails
done
clear
C)
Nictitating membrane
done
clear
D)
Vermiform appendix
done
clear
View Answer play_arrow
question_answer 140) The chemical used in 'National Malaria Eradication Programme' is
A)
2,4-D
done
clear
B)
BHC
done
clear
C)
DDT
done
clear
D)
Pyrethroid
done
clear
View Answer play_arrow
question_answer 141) An eukaryotic gene contains two kinds of base sequences. Which of these plays an important role in protein synthesis ?
A)
Introns
done
clear
B)
Exons
done
clear
C)
Both and
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 142) The Leydig cells secrete
A)
oestrogen
done
clear
B)
testosterone
done
clear
C)
progesterone
done
clear
D)
corticosterone
done
clear
View Answer play_arrow
question_answer 143) The function of pineal body is to
A)
lighten the skin colours
done
clear
B)
control sexual behavior
done
clear
C)
regulate the period of puberty
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 144) Which of the following nerve is purely motor nerve ?
A)
Vagus
done
clear
B)
Facial
done
clear
C)
Abducens
done
clear
D)
Trigeminal
done
clear
View Answer play_arrow
question_answer 145) Which of the following parts of a neuron is covered by fatty sheath ?
A)
Axon
done
clear
B)
Cyton
done
clear
C)
Dendrite
done
clear
D)
Node of Ranvier
done
clear
View Answer play_arrow
question_answer 146) The enzyme, which combines with non-protein part to form a functional enzyme known as
A)
co-enzyme
done
clear
B)
holoenzyme
done
clear
C)
apoenzyme
done
clear
D)
prosthetic group
done
clear
View Answer play_arrow
question_answer 147) Which of the following enzymes digests protein in stomach?
A)
Trypsin
done
clear
B)
Pepsin
done
clear
C)
Crepsin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 148) Passive food ingestion in Amoeba is known as
A)
import
done
clear
B)
invagination
done
clear
C)
circumfluence
done
clear
D)
circumvallation
done
clear
View Answer play_arrow
question_answer 149) Ecdysone is secreted from
A)
Insects
done
clear
B)
Trematoda
done
clear
C)
Nematoda
done
clear
D)
Polychaeta
done
clear
View Answer play_arrow
question_answer 150) The eggs of silk moth are
A)
haemolecithal
done
clear
B)
telolecithal
done
clear
C)
mesolecithal
done
clear
D)
centrolecithal
done
clear
View Answer play_arrow
question_answer 151) RNA and proteins are formed in
A)
\[{{\text{G}}_{\text{1}}}\text{-}\]phase
done
clear
B)
\[{{\text{G}}_{\text{2}}}\text{-}\]phase
done
clear
C)
S-phase
done
clear
D)
\[{{\text{G}}_{\text{o}}}\text{-}\]phase
done
clear
View Answer play_arrow
question_answer 152) Growth promoting hormone is
A)
\[\text{IAA}\]
done
clear
B)
Gibberellin
done
clear
C)
2,4-D
done
clear
D)
ABA
done
clear
View Answer play_arrow
question_answer 153) Which of the following genes show the heterozygous condition?
A)
Rr
done
clear
B)
RR
done
clear
C)
rr
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 154) Which of the following enzymes is responsible for formation of glucose from glucose-6-phosphate?
A)
Kinase
done
clear
B)
Aldolase
done
clear
C)
Dehydrogenase
done
clear
D)
Phosphatase
done
clear
View Answer play_arrow
question_answer 155) Binomial nomenclature was introduced by
A)
de Vries
done
clear
B)
Carolus Linnaeus
done
clear
C)
Pranti
done
clear
D)
Bentham and Hooker
done
clear
View Answer play_arrow
question_answer 156) Transduction in bacteria was discovered by
A)
Zinder and Lederberg
done
clear
B)
Wallace and Jacob
done
clear
C)
Herelle and Twort
done
clear
D)
Lederberg and Tatum
done
clear
View Answer play_arrow
question_answer 157) Protista includes
A)
unicellular eukaryotes
done
clear
B)
multi cellullar prokaryotes
done
clear
C)
unicellular prokaryotes
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 158) Bakanae disease is caused by
A)
fungus
done
clear
B)
alga
done
clear
C)
bacterium
done
clear
D)
virus
done
clear
View Answer play_arrow
question_answer 159) Fermentation is
A)
anaerobic respiration
done
clear
B)
incomplete oxidation of carbohydrate
done
clear
C)
complete oxidation of carbohydrate
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 160) Lichen is the association of
A)
Protista and algae
done
clear
B)
fungi and bacteria
done
clear
C)
Protista and fungi
done
clear
D)
algae and fungi
done
clear
View Answer play_arrow
question_answer 161) A chemical substance produced by a micro-organism for inhibiting the growth of another is
A)
antibody
done
clear
B)
antibiotic
done
clear
C)
aflatoxin
done
clear
D)
antiallergic
done
clear
View Answer play_arrow
question_answer 162) Which of the following is/are essential fatty acids for man?
A)
Arachidonic acid
done
clear
B)
Linolenic acid
done
clear
C)
Linoleic acid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 163) Golgi apparatus is absent in
A)
higher plant
done
clear
B)
yeast
done
clear
C)
bacteria and blue-green algae
done
clear
D)
liver cells
done
clear
View Answer play_arrow
question_answer 164) Which of the following is the formula of chlorophyll\[\text{- }\!\!\alpha\!\!\text{ }\]?
A)
\[{{\text{C}}_{\text{55}}}{{\text{H}}_{\text{70}}}{{\text{O}}_{\text{2}}}{{\text{N}}_{\text{4}}}\text{Mg}\]
done
clear
B)
\[{{\text{C}}_{\text{55}}}{{\text{H}}_{\text{72}}}{{\text{O}}_{\text{5}}}{{\text{N}}_{\text{4}}}\text{Mg}\]
done
clear
C)
\[{{\text{C}}_{\text{55}}}{{\text{H}}_{\text{72}}}{{\text{O}}_{\text{2}}}{{\text{N}}_{\text{4}}}\text{Mg}\]
done
clear
D)
\[{{\text{C}}_{\text{55}}}{{\text{H}}_{\text{70}}}{{\text{O}}_{\text{5}}}{{\text{N}}_{\text{4}}}\text{Mg}\]
done
clear
View Answer play_arrow
question_answer 165) The largest phase of meiosis\[\text{-I}\] is
A)
prophase\[\text{-I}\]
done
clear
B)
metaphase\[\text{-I}\]
done
clear
C)
anaphase\[\text{-I}\]
done
clear
D)
telophase\[\text{-I}\]
done
clear
View Answer play_arrow
question_answer 166) The number of hydrogen bonds between adenine and thymine is
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 167) Insectivorous plant eats the insect for
A)
Na-K
done
clear
B)
nitrogen
done
clear
C)
chlorine
done
clear
D)
phosphorus
done
clear
View Answer play_arrow
question_answer 168) Which of the following pairs are correctly matched?
A)
Central dogma?codon
done
clear
B)
Okazaki fragments?splicing
done
clear
C)
RNA polymerase?RNA primer
done
clear
D)
Restriction enzyme?Genetic engineering
done
clear
View Answer play_arrow
question_answer 169) Which of the following is most resistant material in pollen grain?
A)
Sporopollenin
done
clear
B)
In tine
done
clear
C)
Cellulose
done
clear
D)
Cuticle
done
clear
View Answer play_arrow
question_answer 170) CO is a pollutant because
A)
it reacts with \[{{C}_{3}}\]
done
clear
B)
it inhibits glycolysis
done
clear
C)
it reacts with haemoglobin
done
clear
D)
it makes nervous system inactive
done
clear
View Answer play_arrow
question_answer 171) First stable product of \[{{C}_{3}}\]-plant is
A)
PGDP
done
clear
B)
PGA
done
clear
C)
oxalic acid
done
clear
D)
malate
done
clear
View Answer play_arrow
question_answer 172) The floral formula Br or EBr \[\otimes \,{{K}_{(5)}}\,{{C}_{(5)}}\,{{A}_{o}}\,{{\overline{G}}_{(3)}}\] belongs to family
A)
Liliaceae
done
clear
B)
Cucurbitacea
done
clear
C)
Graminae
done
clear
D)
Solanaceae
done
clear
View Answer play_arrow
question_answer 173) 'Golden rice' is a rice variety rich in
A)
\[\text{ }\!\!\beta\!\!\text{ -}\]carotene
done
clear
B)
lysine
done
clear
C)
vitamin-C
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 174) Which of the following ecological pyramids never occur in an inverted form?
A)
Pyramid of number
done
clear
B)
Pyramid of biomass
done
clear
C)
Pyramid of energy
done
clear
D)
Pyramid of species richness
done
clear
View Answer play_arrow
question_answer 175) Velamen tissue is found in
A)
mesophytes
done
clear
B)
epiphytes
done
clear
C)
hydrophytes
done
clear
D)
xerophytes
done
clear
View Answer play_arrow
question_answer 176) The edible pan of apple is
A)
mesocarp
done
clear
B)
endocarp
done
clear
C)
epicarp
done
clear
D)
fleshy thalamus
done
clear
View Answer play_arrow
question_answer 177) Active transport
A)
releases energy
done
clear
B)
requires energy
done
clear
C)
produces ATP
done
clear
D)
produces a toxic substance
done
clear
View Answer play_arrow
question_answer 178) Osmosis involves
A)
flow of water without a membrane
done
clear
B)
flow of solute from a semipermeable membrane
done
clear
C)
flow of solvent \[({{H}_{2}}O)\] through semi permeable membrane
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 179) Pollination by insect is
A)
emomophily
done
clear
B)
chiropterophr
done
clear
C)
anemophify
done
clear
D)
zoophily
done
clear
View Answer play_arrow
question_answer 180) Virus multiplies in
A)
soil
done
clear
B)
dead tissue
done
clear
C)
living tissue
done
clear
D)
culture medium
done
clear
View Answer play_arrow
question_answer 181) Which of the following techniques, other than microscopy is used for study of cell?
A)
Maceration
done
clear
B)
Plasmolysis
done
clear
C)
Chromatography
done
clear
D)
Autoradiography
done
clear
View Answer play_arrow
question_answer 182) Robert Hooke used the term cell in the year
A)
1650
done
clear
B)
1665
done
clear
C)
1865
done
clear
D)
1960
done
clear
View Answer play_arrow
question_answer 183) Protein synthesis takes place in
A)
ribosomes
done
clear
B)
chloroplasts
done
clear
C)
mitochondria
done
clear
D)
Golgi bodies
done
clear
View Answer play_arrow
question_answer 184) Replication of centriole occurs during
A)
interphase
done
clear
B)
prophase
done
clear
C)
late prophase
done
clear
D)
late telophase
done
clear
View Answer play_arrow
question_answer 185) Which of the following is true about bryophytes?
A)
They are thalloid
done
clear
B)
They contain chloroplast
done
clear
C)
They possess archegonia
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 186) The kidney-shaped covering of sorus in Dryopteris's, is called
A)
placenta
done
clear
B)
ramentum
done
clear
C)
sporophyll
done
clear
D)
indusium
done
clear
View Answer play_arrow
question_answer 187) Replum is found in family
A)
Labiatae
done
clear
B)
Malvaceae
done
clear
C)
Compositae
done
clear
D)
Brassicaceae
done
clear
View Answer play_arrow
question_answer 188) Which of the following is a wheat fruit?
A)
Achene
done
clear
B)
Cypsella
done
clear
C)
Caryopsis
done
clear
D)
Endosperm
done
clear
View Answer play_arrow
question_answer 189) A gymnospermic leaf carries 16 chromosomes. The number of chromosomes in its endosperm is
A)
24
done
clear
B)
16
done
clear
C)
12
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 190) Tyloses thickenings are seen in
A)
collenchyma
done
clear
B)
phloem cells
done
clear
C)
ray parenchyma only
done
clear
D)
ray parenchyma and xylem cells
done
clear
View Answer play_arrow
question_answer 191) Ethyl alcohol is commercially manufactured from
A)
bajra
done
clear
B)
grapes
done
clear
C)
maize
done
clear
D)
sugarcane
done
clear
View Answer play_arrow
question_answer 192) A hormone delaying senescence is
A)
auxin
done
clear
B)
cytokinin
done
clear
C)
ethylene
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 193) What name has been assigned to the genus produced by a cross between cabbage and radish?
A)
Secale
done
clear
B)
Bursa pastoris
done
clear
C)
Lysogenicophyll
done
clear
D)
Raphanobrassica
done
clear
View Answer play_arrow
question_answer 194) Morphine obtained from Opium is
A)
latex
done
clear
B)
pome
done
clear
C)
alkaloid
done
clear
D)
tannin
done
clear
View Answer play_arrow
question_answer 195) The term heterosis was first coined by
A)
McCtintock
done
clear
B)
Power
done
clear
C)
Swaminathan
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 196) L-shaped chromosomes are also called
A)
acrocentric
done
clear
B)
telocentric
done
clear
C)
sub-metacentric
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 197) Which of the following is/are grouped under phanerogams ?
A)
Angiospenns
done
clear
B)
Gymnosperms
done
clear
C)
Pteridophytes
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 198) A bacterium divides after every 35 minutes. If culture containing \[{{10}^{5}}\] cells per mL is grown then cell concentration per mL after 175 minutes will be
A)
\[175\times {{10}^{5}}\]
done
clear
B)
\[125\times {{10}^{5}}\]
done
clear
C)
\[48\times {{10}^{5}}\]
done
clear
D)
\[32\times {{10}^{5}}\]
done
clear
View Answer play_arrow
question_answer 199) Cladonia rangiferina is a/an
A)
algae
done
clear
B)
lichen
done
clear
C)
fungus
done
clear
D)
angiosperm
done
clear
View Answer play_arrow
question_answer 200) Which of the following is an algal parasite?
A)
Volvox
done
clear
B)
Ulothrix
done
clear
C)
Porphyra
done
clear
D)
Cephaleuros
done
clear
View Answer play_arrow