A) \[NO_{3}^{-}\]
B) \[NO_{2}^{-}\]
C) \[N{{O}_{2}}\]
D) \[NO_{2}^{+}\]
Correct Answer: D
Solution :
In \[NO_{3}^{-}\], N atom undergoes \[s{{p}^{2}}\]-hybridisation. Thus, the ion has trigonal planar geometry with bond angle \[{{120}^{o}}C\].
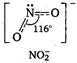
You need to login to perform this action.
You will be redirected in
3 sec
