A) phenol is more soluble in polar solvents
B) alcohol does not lose hydrogen atom
C) phenoxide ion is stabilised by resonance
D) phenoxide ion do not exhibit resonance
Correct Answer: C
Solution :
Key Idea: Here is resonance. Due to resonance the phenoxide ion is more stable whereas resonance is not possible in alkoxide ion.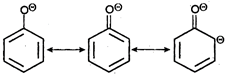
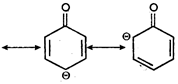 \[R-{{O}^{O-}}\] no resonance is possible, Since, phenoxide ion is better stabilised by resonance, the phenol has more tendency to form phenoxide ion by releasing \[{{H}^{+}}\]ion. So, phenols are acidic in nature.
\[R-{{O}^{O-}}\] no resonance is possible, Since, phenoxide ion is better stabilised by resonance, the phenol has more tendency to form phenoxide ion by releasing \[{{H}^{+}}\]ion. So, phenols are acidic in nature.
You need to login to perform this action.
You will be redirected in
3 sec
