A) triangular planar
B) pyramidal
C) T-shape
D) trigonal bipyramidal
Correct Answer: C
Solution :
\[\text{Cl}{{\text{F}}_{3}}=3\]bond pairs + 2 lone pairs \[\Rightarrow \] 5 hybrid orbitals So, the shape should be trigonal bipyramidal. But, the shape becomes T-shape because of the presence of two lone pairs of electrons.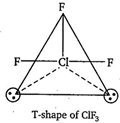
You need to login to perform this action.
You will be redirected in
3 sec
