A) \[{{[Sc{{(CN)}_{6}}]}^{3-}}\]
B) \[{{[Co{{(CN)}_{6}}]}^{3-}}\]
C) \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
D) \[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
Correct Answer: D
Solution :
Key Idea: Calculate the oxidation state of central metal ion as that gives idea of about number of unpaired or paired electrons. \[Sc=21=1{{s}^{2}}{{,}^{2}}{{s}^{2}}{{,}^{2}}{{p}^{6}}{{,}^{3}}{{s}^{2}},3{{p}^{6}},{{\,}^{4}}{{s}^{2}},{{\,}^{3}}{{d}^{1}}\]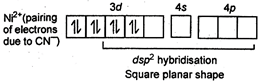 No unpaired electron hence, diamagnetic \[{{[Co{{(CN)}_{6}}]}^{3-}}\]
No unpaired electron hence, diamagnetic \[{{[Co{{(CN)}_{6}}]}^{3-}}\] You need to login to perform this action.
You will be redirected in
3 sec
