A) \[NO_{3}^{-}\]
B) \[O_{2}^{-}\]
C) \[A{{g}^{+}}\]
D) \[{{K}^{+}}\]
Correct Answer: C
Solution :
When aqueous solution of \[\text{AgN}{{\text{O}}_{\text{3}}}\]is added to solution, positively charged sol of \[\text{AgI}\]is obtained due to the adsorption of \[\text{A}{{\text{g}}^{+}}\]ions on \[\text{AgI}\]molecules.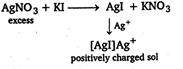
You need to login to perform this action.
You will be redirected in
3 sec
