A) 2, 2-bipyridyl
B) DMG
C) Ethylenediamine
D) None of these
Correct Answer: D
Solution :
Key Idea: Hexadentate ligand donates 6 pair of electrons to central atom. (a) 2, 2-dipyridyl-bidentate ligand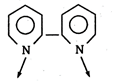 (b) DMG- bidentate ligand \[\begin{align} & C{{H}_{3}}-C=N-O\xrightarrow{{}} \\ & C{{H}_{3}}-\overset{|}{\mathop{C}}\,=\underset{OH}{\mathop{\underset{|}{\mathop{N}}\,}}\,\xrightarrow{{}} \\ \end{align}\] (c) Ethylenediamine- pentadentate ligand \[\therefore \] None of the given ligand is hexadentate ligand.
(b) DMG- bidentate ligand \[\begin{align} & C{{H}_{3}}-C=N-O\xrightarrow{{}} \\ & C{{H}_{3}}-\overset{|}{\mathop{C}}\,=\underset{OH}{\mathop{\underset{|}{\mathop{N}}\,}}\,\xrightarrow{{}} \\ \end{align}\] (c) Ethylenediamine- pentadentate ligand \[\therefore \] None of the given ligand is hexadentate ligand.
You need to login to perform this action.
You will be redirected in
3 sec
