A) monobasic
B) dibasic
C) tribasic
D) tetrabasic
Correct Answer: C
Solution :
Key Idea: For oxyacids no. of OH groups in structure = basicity Orthophosphoric acid is \[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}\]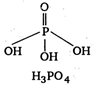 \[\because \] \[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}\]has 3- OH groups in structure \[\therefore \]It is tribasic acid.
\[\because \] \[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}\]has 3- OH groups in structure \[\therefore \]It is tribasic acid.
You need to login to perform this action.
You will be redirected in
3 sec
