A) \[cis-\]1, 2-dichloroethylene
B) trans-1, 2- dichloroethylene
C) 1, 1- dichloroethylene
D) none of the above
Correct Answer: B
Solution :
Key Idea: Dipole moment is a vector quantity. The molecules having symmetrical structure have zero dipole moment. (a)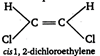 (b)
(b) 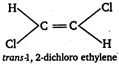 (c)
(c) 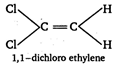 \[\because \] Out of given choices, only irons 1, 2-dichloro ethylene is symmetrical. \[\therefore \] It has zero dipole moment.
\[\because \] Out of given choices, only irons 1, 2-dichloro ethylene is symmetrical. \[\therefore \] It has zero dipole moment.
You need to login to perform this action.
You will be redirected in
3 sec
