| Note: - (\[{{\Delta }_{o}}=\] crystal field splitting energy in an octahedral field, P = electron pairing energy) |
A) \[\frac{-12}{5}{{\Delta }_{o}}+p\]
B) \[\frac{-12}{5}{{\Delta }_{o}}+3p\]
C) \[\frac{-2}{5}{{\Delta }_{o}}+2p\]
D) \[\frac{-2}{5}{{\Delta }_{o}}+p\]
Correct Answer: A
Solution :
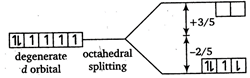 \[\therefore \] Total energy \[={{\Delta }_{o}}+P\] \[=\left( +\frac{3}{5}\times 0-\frac{2}{5}\times 6 \right){{\Delta }_{o}}+P\] \[=\frac{-12}{5}{{\Delta }_{o}}+P\]
\[\therefore \] Total energy \[={{\Delta }_{o}}+P\] \[=\left( +\frac{3}{5}\times 0-\frac{2}{5}\times 6 \right){{\Delta }_{o}}+P\] \[=\frac{-12}{5}{{\Delta }_{o}}+P\]
You need to login to perform this action.
You will be redirected in
3 sec
