A) \[{{[Ti{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
B) \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
C) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
D) \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
Correct Answer: B
Solution :
[a] Electronic configuration of \[\text{T}{{\text{i}}^{\text{3+}}}\] in \[{{[Ti{{(N{{H}_{3}})}_{6}}]}^{3+}}\]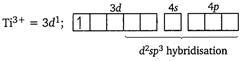 [b] Electronic configuration of \[\text{C}{{\text{r}}^{\text{3+}}}\]in\[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
[b] Electronic configuration of \[\text{C}{{\text{r}}^{\text{3+}}}\]in\[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\] 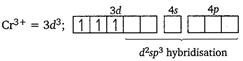 [c] Electronic configuration of \[\text{C}{{\text{O}}^{\text{3+}}}\]in\[{{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\text{:}\]
[c] Electronic configuration of \[\text{C}{{\text{O}}^{\text{3+}}}\]in\[{{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\text{:}\] 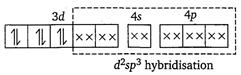 [d] Electronic configuration of\[Z{{n}^{2+}}\] in \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}:\]
[d] Electronic configuration of\[Z{{n}^{2+}}\] in \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}:\] 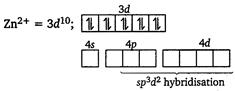 \[\therefore \]\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\]is an outer orbital complex and is diamagnetic.
\[\therefore \]\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\]is an outer orbital complex and is diamagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
