A) \[{{\text{I}}_{\text{2}}}\] will be reduced to \[{{\text{I}}^{-}}\]
B) There will be no redox reaction
C) \[{{\text{I}}^{-}}\] will be oxidised to \[{{\text{I}}_{\text{2}}}\]
D) \[\text{F}{{\text{e}}^{\text{2+}}}\]will be oxidised to \[\text{F}{{\text{e}}^{\text{3+}}}\]
Correct Answer: C
Solution :
\[2{{I}^{-}}\xrightarrow[{}]{{}}{{I}_{2}}+2{{e}^{-}}\](Oxidation half-reaction) \[E_{oxi.}^{o}=-0.536\,V.\] \[F{{e}^{3+}}+{{e}^{-}}\xrightarrow[{}]{{}}F{{e}^{2+}}\](Reduction half-reaction)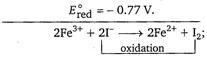 \[{{E}^{o}}={{E}^{o}}_{oxi}+{{E}^{o}}_{red}\] \[=+ve\] So, reaction will take place.
\[{{E}^{o}}={{E}^{o}}_{oxi}+{{E}^{o}}_{red}\] \[=+ve\] So, reaction will take place.
You need to login to perform this action.
You will be redirected in
3 sec
