A) 3
B) 4
C) 5
D) 2
Correct Answer: A
Solution :
\[E=K{{E}_{\max }}+W\] \[=e{{V}_{0}}+W\] \[=10+2.75\] \[E=12.75\,eV\]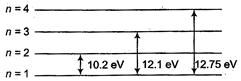 Difference of 4 and 1 energy level is 12.75 eV. So, higher energy level is 4 to ground and excited state is n = 3.
Difference of 4 and 1 energy level is 12.75 eV. So, higher energy level is 4 to ground and excited state is n = 3.
You need to login to perform this action.
You will be redirected in
3 sec
