A) Dissimilar in hybridisation for the central atom with different structures
B) Isostructural with same hybridisation for the central atom
C) Isostructural with different hybridization for the central atom
D) Similar in hybridisation for the central atom with different structures
Correct Answer: A
Solution :
In\[N{{O}_{3}}\], \[H=\frac{1}{2}[5+0-0+1]=3\] Thus, in \[\text{NO}_{3}^{-},\] the central atom is sp2 hybridized and it has trifocal planar geometry.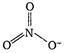 In \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}},\] \[H=\frac{1}{2}[6+3-1+0]=4\] Thus, O is sp3 hybridised in \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\] and \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\] has pyramidal geometry due to the presence of one lone pair of electrons.
In \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}},\] \[H=\frac{1}{2}[6+3-1+0]=4\] Thus, O is sp3 hybridised in \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\] and \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\] has pyramidal geometry due to the presence of one lone pair of electrons. You need to login to perform this action.
You will be redirected in
3 sec
