A) \[CaC{{O}_{3}}\]
B) \[N{{a}_{2}}C{{O}_{3}}\]
C) \[{{K}_{2}}C{{O}_{3}}\]
D) \[CaS{{O}_{4}}\cdot 2{{H}_{2}}O\]
Correct Answer: A
Solution :
Given,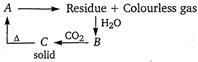 Thus, the compound 'A' must be \[\text{CaC}{{\text{O}}_{\text{3}}}\]and the reactions are as follows
Thus, the compound 'A' must be \[\text{CaC}{{\text{O}}_{\text{3}}}\]and the reactions are as follows 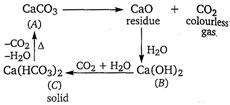
You need to login to perform this action.
You will be redirected in
3 sec
