-
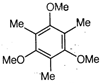
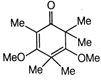
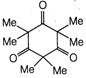
question_answer1) A particle is projected with an angle of projection \[\theta \] to the horizontal line passing? through the points (P, Q) and (Q, P) referred to horizontal and vertical axes (can be treated as x-axis and y-axis respectively). The angle of projection can be given by
A)
\[[L\,\,{{T}^{-1}}{{Q}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}\,\,{{T}^{-1}}{{Q}^{-2}}]\]
done
clear
C)
\[\left[ \text{LT}{{\text{Q}}^{-\text{1}}} \right]\]
done
clear
D)
\[{{O}_{1}}\]
done
clear
View Answer play_arrow
-
question_answer2)
Determine the height above the dashed line \[{{O}_{2}}\]attained by the water stream coming out through the hole is situated at point B in the diagram given below. Given that \[{{O}_{1}}\] 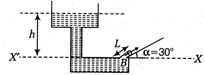
A)
10m
done
clear
B)
7.1m
done
clear
C)
5 m
done
clear
D)
3.2 m
done
clear
View Answer play_arrow
-
question_answer3) If the magnetizing field on a ferromagnetic material is increased, its permeability
A)
decreased
done
clear
B)
increased
done
clear
C)
is unaffected
done
clear
D)
may be increased or decreased
done
clear
View Answer play_arrow
-
question_answer4) A ball is dropped from a bridge 122.5m above a river. After the ball has been falling for 2s, a second ball is thrown straight down after it. What must the initial velocity of the second ball be so that both hit the water at the same time?
A)
40m/s
done
clear
B)
55.5m/s
done
clear
C)
26.1 m/s
done
clear
D)
9.6 m/s
done
clear
View Answer play_arrow
-
question_answer5) A body of mass 40 kg resting on rough horizontal surface is subjected to a force P which is just enough to start the motion of the body. If \[{{O}_{2}}\]and the force P is continuously applied on the body, then acceleration of the body is
A)
zero
done
clear
B)
\[\left( \text{given},\text{hc=124}0\text{ eV}-\text{nm},\text{e}=\text{1}.\text{6}\times \text{1}{{0}^{-\text{19}}}\text{ C} \right)\]
done
clear
C)
\[5\mu A\]
done
clear
D)
\[40\mu A\]
done
clear
View Answer play_arrow
-
question_answer6) The self inductance of a coil having 500 turns is\[50\text{ }mH\]. The magnetic flux through the cross-sectional area of the coil while current through it is \[8\text{ }mA\] is found to be
A)
\[50\mu A\]
done
clear
B)
\[114\mu A\]
done
clear
C)
\[{{l}_{1}}\]
done
clear
D)
\[{{T}_{1}}\]
done
clear
View Answer play_arrow
-
question_answer7) A uniform metallic rod rotates about its perpendicular bisector with constant angular speed. If it is heated uniformly to raise its temperature slightly, then
A)
its speed of rotation increases
done
clear
B)
its speed of rotation decreases
done
clear
C)
its speed of rotation remains same
done
clear
D)
its speed increases because its moment of inertia Increases
done
clear
View Answer play_arrow
-
question_answer8) A uniform disc is acted by two equal forces of magnitude F. One of them, acts tangentially to the disc, while other one is acting at the central point of the disc. The friction between disc surface and ground surface is \[nF\]. If r be the radius of the disc, then the value of n would be (in N)
A)
0
done
clear
B)
1.2
done
clear
C)
2.0
done
clear
D)
3.2
done
clear
View Answer play_arrow
-
question_answer9) While keeping area of cross-section of a solenoid same, the number of turns and length of solenoid one both doubled. The self inductance of the coil will be
A)
halved
done
clear
B)
doubted
done
clear
C)
\[{{l}_{2}}\] times the original value
done
clear
D)
unaffected
done
clear
View Answer play_arrow
-
question_answer10)
Consider the circular loop having current\[{{T}_{2}}\] and with central point O. The magnetic field at the central point O is 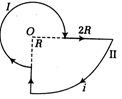
A)
\[\frac{{{l}_{1}}+{{l}_{2}}}{2}\] acting downward
done
clear
B)
\[\sqrt{{{l}_{1}}+{{l}_{2}}}\] acting downward
done
clear
C)
\[\frac{{{l}_{1}}{{T}_{2}}-{{l}_{2}}{{T}_{1}}}{{{T}_{2}}-{{T}_{1}}}\] acting downward
done
clear
D)
\[\frac{{{l}_{1}}{{T}_{2}}+{{l}_{2}}{{T}_{1}}}{{{T}_{1}}+{{T}_{2}}}\] acting upward
done
clear
View Answer play_arrow
-
question_answer11) The Boolean expression\[\frac{v\,dv}{dX}=-{{\omega }^{2}}\text{x with the initial condition V}={{\text{V}}_{0}}\text{ at}\], where P and Q are the inputs of the logic circuit, represents
A)
AND gate
done
clear
B)
NAND gate
done
clear
C)
NOT gate
done
clear
D)
OR gate
done
clear
View Answer play_arrow
-
question_answer12)
Consider the diagram shown below. 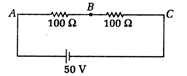 A voltmeter of resistance \[150\Omega \]. is connected across A and 5. The potential drop across and C measured by voltmeter is
A voltmeter of resistance \[150\Omega \]. is connected across A and 5. The potential drop across and C measured by voltmeter is
A)
29V
done
clear
B)
27V
done
clear
C)
31 V
done
clear
D)
30V
done
clear
View Answer play_arrow
-
question_answer13) Two spherical nuclei have mass numbers 2l6 and 64 with their radii \[V=\sqrt{v_{0}^{2}+{{\omega }^{2}}{{X}^{2}}}\] and \[V=\sqrt{v_{0}^{2}-{{\omega }^{2}}{{X}^{2}}}\]respectively The ratio, \[V=\sqrt[3]{v_{0}^{3}+{{\omega }^{3}}{{X}^{3}}}\] is equal to
A)
\[3:2\]
done
clear
B)
\[1:3\]
done
clear
C)
\[1:2\]
done
clear
D)
\[2:3\]
done
clear
View Answer play_arrow
-
question_answer14)
A massless rod S having length 27 has equal point masses attached to its two ends as shown in figure. The rod is rotating about an axis passing through its centre and making angle \[\alpha \]with the axis. The magnitude of change of momentum of rod i.e. \[V={{v}_{0}}-{{({{\omega }^{3}}{{X}^{3}}{{e}^{{{X}^{3}}}})}^{1/3}}\] equals 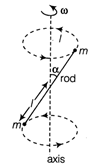
A)
\[\frac{2\,{{m}^{2}}}{3}g\]
done
clear
B)
\[\frac{4\,{{m}^{2}}}{3}g\]
done
clear
C)
\[\frac{\,{{m}^{2}}}{\sqrt{2}}g\]
done
clear
D)
\[5\Omega \]
done
clear
View Answer play_arrow
-
question_answer15) A semiconductor having electron and hole mobilities \[2A\] and \[\frac{12}{7}A\] respectively if its intrinsic carrier density is\[{{n}_{i}},\]then what will be the value of hole concentration P for which the conductivity will be minimum at a given temperature?
A)
\[\omega =\sqrt{rg\,\sin \theta }\]
done
clear
B)
\[\omega =\sqrt{g/r\,\,\cos \theta }\]
done
clear
C)
\[\omega =\sqrt{\frac{gr}{\cos \theta }\,\,}\]
done
clear
D)
\[\omega =\sqrt{\frac{gr}{\tan \theta }\,\,}\]
done
clear
View Answer play_arrow
-
question_answer16) In terms of basic units of mass (M), length (L), time (T) and charge (Q), the dimensions of magnetic permeability of vacuum (Ho) would be
A)
\[\pi /2\]
done
clear
B)
\[\pi /3\]
done
clear
C)
\[-\pi /6\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
-
question_answer17) The black body spectrum of an object \[250\mu F\] is such that its radiant intensity (i.e. intensity per unit wavelength interval) is maximum at a wavelength of 200 nm. Another object \[20\Omega \] has the maximum radiant intensity at 600 nm. The ratio of power emitted per unit area by source \[9\times {{10}^{4}}\,\,Hz\]to that of source \[16\times {{10}^{7}}\,\,Hz\] is
A)
\[1:81\]
done
clear
B)
\[1:9\]
done
clear
C)
\[9:1\]
done
clear
D)
\[81:1\]
done
clear
View Answer play_arrow
-
question_answer18) A beam of light of wavelength 400 nm and power\[1.55\text{ }mW\]is directed at the cathode of a photoelectric cell. If only 10% of the incident photons effectively produce photoelectron, then find current due to these electrons. \[8\times {{10}^{5}}\,\,Hz\]
A)
\[9\times {{10}^{3}}\,\,Hz\]
done
clear
B)
\[\lambda \,~\text{is greater than 2d}\]
done
clear
C)
\[\lambda \,quals2d\]
done
clear
D)
\[\lambda \,\text{is smaller than or equal to 2d}\]
done
clear
View Answer play_arrow
-
question_answer19) The molar specific heat of a gas as given from the kinetic theory is - R. If it is not specified whether it is \[{{C}_{p}}\]or \[{{C}_{v}},\] one could conclude that the molecules of the gas
A)
are definitely monoatomic
done
clear
B)
are definitely rigid diatomic
done
clear
C)
are definitely non-rigid diatomic
done
clear
D)
can be monoatomic or rigid diatomic
done
clear
View Answer play_arrow
-
question_answer20) The length of a metal wire is \[\lambda \,\,\text{is smaller than 2d}\] when the tension in it is \[a=\frac{{{C}^{2}}{{B}^{2}}l-F}{m}\] and is \[a=\frac{F}{m+CBI}\] when the tension is \[a=\frac{F{{C}^{2}}{{B}^{2}}l}{m}\]. The natural length of the wire is
A)
\[a=\frac{F}{m+{{C}^{2}}{{B}^{2}}l}\]
done
clear
B)
\[\left( \text{take g }=\text{ 1}0\text{ m}/{{\text{s}}^{\text{2}}} \right)\]
done
clear
C)
\[1.4\times {{10}^{-4}}\text{J}\]
done
clear
D)
\[0.75\times {{10}^{-3}}\text{J}\]
done
clear
View Answer play_arrow
-
question_answer21) The velocity vector v and displacement vector x of a particle executing SHM are related as \[5.75\times {{10}^{-3}}\text{J}\]x = 0. The velocity v, when displacement is x, is
A)
\[4.9\times {{10}^{-5}}\text{J}\]
done
clear
B)
\[\text{F}=\text{1}0+0.\text{5x}\]
done
clear
C)
\[(\text{take},\text{g}=0\text{m}/{{\text{s}}^{\text{2}}}\]
done
clear
D)
\[R=6.4\times {{10}^{3}}m\]
done
clear
View Answer play_arrow
-
question_answer22)
consider the diagram shown below in which two masses of m and 2m are placed on a fixed triangular wedge. 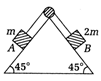 The coefficient of friction between block A and the wedge is 2/3, while that for block B and the wedge is 1/3. If the whole system is released from rest, then acceleration of block A is
The coefficient of friction between block A and the wedge is 2/3, while that for block B and the wedge is 1/3. If the whole system is released from rest, then acceleration of block A is
A)
Zero
done
clear
B)
\[\text{1}0\text{m}{{\text{s}}^{\text{-1}}}\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
View Answer play_arrow
-
question_answer23)
In the arrangement shown in figure, the current through \[{{\tan }^{-1}}\left( \frac{4}{3} \right)\]resistor is 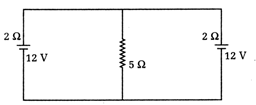
A)
\[{{\cos }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
B)
Zero
done
clear
C)
\[[M{{L}^{3}}{{l}^{-1}}{{T}^{-3}}]\]
done
clear
D)
\[[{{M}^{2}}{{L}^{2}}{{l}^{-1}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
-
question_answer24) A hemispherical bowl of radius r is set rotating about its axis of symmetry in vertical. A small block kept in the bowl rotates with the bowl without slipping on its surface. If the surface of the bowl is smooth and the angle made by the radius through the block with the vertical is \[\theta \], then find the angular speed at which the ball is rotating.
A)
\[[M{{L}^{3}}{{l}^{1}}{{T}^{-3}}]\]
done
clear
B)
\[[M{{L}^{-3}}{{l}^{-1}}{{T}^{-3}}]\]
done
clear
C)
\[\sqrt{\frac{{{M}_{p}}}{{{M}_{e}}}}\]
done
clear
D)
\[\sqrt{\frac{{{M}_{e}}}{{{M}_{p}}}}\]
done
clear
View Answer play_arrow
-
question_answer25) The phase difference between the flux linked with a coil rotating in a uniform magnetic field and induced emf produced in it is
A)
\[f\]
done
clear
B)
\[({{f}_{pp}})\]
done
clear
C)
\[({{f}_{pn}})\]
done
clear
D)
\[({{f}_{nn}})\]
done
clear
View Answer play_arrow
-
question_answer26) A condenser of\[{{f}_{pp}}<{{f}_{pn}}={{f}_{nn}}\]is connected in parrallel to a coil of inductance\[0.16\text{ }mH\] while its effective resistance is \[(\mu )\] Determine the resonant frequency.
A)
\[\mu \propto n\]
done
clear
B)
\[{{m}_{1}}\]
done
clear
C)
\[{{m}_{2}}\]
done
clear
D)
\[\frac{2a}{1.22\lambda }\]
done
clear
View Answer play_arrow
-
question_answer27) The variation of magnetic susceptibility with the temperature of a ferromagnetic material can be ploted as
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer28) For Bragg's diffraction by a crystal to occur, then the X-ray of wavelength X and interatomic distance d must be
A)
\[\cos \theta =\frac{2R}{\sqrt{{{R}^{2}}+\omega {{L}^{2}}}}\]
done
clear
B)
\[\frac{4}{5}r\]
done
clear
C)
\[h/4\]
done
clear
D)
\[\lambda /4\]
done
clear
View Answer play_arrow
-
question_answer29)
A wire having mass m and length 1 can freely slide on a pair of parallel smooth horizontal rails placed in vertical magnetic field B. The rails are connected by a capacitor of capacitance C. The electric resistance of the rails and the wire is zero. If a constant force F acts on the wire as shown in the figure. Then, the acceleration of the wire can be given as 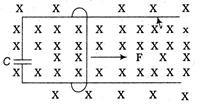
A)
\[\left( \text{kJ mo}{{\text{l}}^{\text{-1}}} \right)\]
done
clear
B)
\[\left( \text{kJ mo}{{\text{l}}^{\text{-1}}} \right)\]
done
clear
C)
\[{{\left[ \text{Co}{{\left( \text{N}{{\text{H}}_{\text{3}}} \right)}_{\text{4}}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\]
done
clear
D)
\[\text{I}\]
done
clear
View Answer play_arrow
-
question_answer30)
Consider the situation shown in figure A spring of spring constant 400 N/m is attached at one end to a wedge fixed rigidly with the horizontal part. A 40 g mass is released from rest while situated at a height 5 cm the curved track. The minimum deformation in the spring is nearly equal to \[\text{II}\] 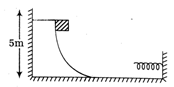
A)
9.8 m
done
clear
B)
9.8 cm
done
clear
C)
.98m,
done
clear
D)
.009km
done
clear
View Answer play_arrow
-
question_answer31) A block having mass 227 collides with an another stationary block having mass 2m. The lighter block comes to rest after collision. If the velocity of first block is v, then the value of coefficient of restitution will must be
A)
0.5
done
clear
B)
0.4
done
clear
C)
0.6
done
clear
D)
0.8
done
clear
View Answer play_arrow
-
question_answer32) A uniform sphere of mass 500 g rolls without slipping on a plane surface so that its centre moves at a speed of 0.02 m/s. The total kinetic energy of rolling sphere would be (in J)
A)
\[\text{III}\]
done
clear
B)
\[\text{IV}\]
done
clear
C)
\[\text{A=I,B=IV}\]
done
clear
D)
\[\text{A=IV,B=I}\]
done
clear
View Answer play_arrow
-
question_answer33) The force on a particle as the function of displacement x (in x-direction) is given by \[\text{A=III,B=II}\] The work done corresponding to displacement of particle from\[x=0\]to\[x=2\] unit is
A)
25J
done
clear
B)
29J
done
clear
C)
21J
done
clear
D)
18J
done
clear
View Answer play_arrow
-
question_answer34) The reading of a spring balance corresponds to 100 N while situated at the north pole and a body is kept on it. The weight record on the same scale if it is shifted to the equator, is \[\text{A=II,B=III}\]and radius of the earth, \[{{[Fe{{({{H}_{2}}O)}_{5}}NO]}^{2+}}\]
A)
99.66 N
done
clear
B)
110N
done
clear
C)
97.66 N
done
clear
D)
106N
done
clear
View Answer play_arrow
-
question_answer35) If the intensity is increased by a factor of 20, then how many decibels in the sound level increased?
A)
18
done
clear
B)
13
done
clear
C)
9
done
clear
D)
7
done
clear
View Answer play_arrow
-
question_answer36) On the same path, the source and observer are moving such a ways that the distance between these two increases with the time. The speeds of source and observer are same and equal to \[NO_{3}^{-}\] with respect to the ground while no wind is blowing. The apparent frequency received by observer is 1950 Hz, then the original frequency must be (the speed of sound in present medium is 340 m/s)
A)
2068 Hz
done
clear
B)
2100 Hz
done
clear
C)
1903 Hz
done
clear
D)
602 Hz
done
clear
View Answer play_arrow
-
question_answer37)
Consider the ray diagram for the refraction given below. The maximum value of angle 9 for which the light suffers total internal reflection at the vertical surface, is 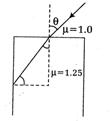
A)
\[\text{Fe(I)}\,\,\text{and}\,\,\text{NO}\,\,\text{as}\,N{{O}^{+}}\]
done
clear
B)
\[C=20%,H=6.67%\]
done
clear
C)
\[N=46.67%\]
done
clear
D)
\[X\]
done
clear
View Answer play_arrow
-
question_answer38) The near point and far point of a person are 40 cm and 250 cm respectively. Determine the power of the lens he/she should use while reading a book kept at distance 25 cm from the eye.
A)
2.5D
done
clear
B)
5.0D
done
clear
C)
1.5D
done
clear
D)
3.5D
done
clear
View Answer play_arrow
-
question_answer39) The dimensional formula for electric flux is
A)
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CON}{{\text{H}}_{\text{2}}}\]
done
clear
B)
\[\text{C}{{\text{H}}_{\text{3}}}NCO\]
done
clear
C)
\[\text{C}{{\text{H}}_{\text{3}}}\text{CON}{{\text{H}}_{2}}\]
done
clear
D)
\[{{(N{{H}_{2}})}_{2}}CO\]
done
clear
View Answer play_arrow
-
question_answer40) An electron of mass Mg, initially at rest, moves through a certain distance in a uniform electric field in time \[{{t}_{1}}\]. A proton of mass \[{{M}_{p}}\] also intially at rest, takes time \[{{t}_{2}}\] to move through an equal distance in this uniform electric field. Neglecting the effect of gravity, the ratio \[{{t}_{2}}/{{t}_{1}}\]is nearly equal to
A)
1
done
clear
B)
\[\text{K}{{\text{O}}_{\text{2}}}\]
done
clear
C)
\[\text{C}{{\text{O}}_{\text{2}}}\]
done
clear
D)
1836
done
clear
View Answer play_arrow
-
question_answer41) Assertion (A) In an elastic collision between two bodies, the relative speed of the bodies after collision is equal to the relative speed before the collision. Reason (R) In an elastic collision, the linear momentum of the system is conserved. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer42) Assertion (A) If there is no external torque on a body about its centre of mass, then the velocity of the centre of mass remains constant. Reason (R) The linear momentum of an isolated system remains constant. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer43) Assertion (A) An astronaut in an orbiting space station above the earth experience weightlessness. Reason (R) An object moving around the earth under the influence of earth's gravitational force is in a state of 'free fall'. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer44) Assertion (A) The stream of water flowing at high speed from a garden hose, pipe tends to spread like a fountain when held vertically up but tends to narrow down when held vertically down. Reason (R) In any steady flow of an incompressible fluid, the volume flow rate of the fluid remains constant. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer45) Assertion (A) The total translational kinetic energy of all the molecules of a given mass of an ideal gas is 1.5 times the product of its pressure and volume. Reason (R) The molecules of gas collide with each other and the velocities of the molecules change due to the collision. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer46) Assertion (A) The relation among u, v and \[\text{C}{{\text{O}}_{\text{2}}}\] for the spherical mirror is valid only for mirrors whose sizes are very small compared to their radii of curvature. Reason (R) The laws of reflection are strictly valid for plane surfaces but not for large spherical surfaces. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer47) Assertion (A) In a meter bridge experiment, null point for an unknown resistance is put inside an enclosure maintained at a higher temperature. The null point can be obtained at the same point as before by decreasing the value of the standard resistance. Reason (R) Resistance of metal increases with increase in temperature Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer48) Assertion (A) Forces acting between proton-proton\[{{\text{O}}_{\text{2}}}\], proton-neutron \[{{S}_{N}}1\] and neutron-neutron \[benzyl>allyl>{{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }}>Me\] are such that \[Me>{{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }}>allyl>benzyl\] Reason (R) Electrostatic force of repulsion between two protons reduces net nuclear forces between them. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer49) Assertion (A) The magnetic moment \[{{3}^{\circ }}>{{2}^{\circ }}>{{1}^{\circ }}>Me>allyl>benzyl\] of e an electron revolving around the nucleus decreases with increasing principle quantum number (n). Reason (R) Magnetic moment of the revolving electron \[benzyl>allyl>{{3}^{\circ }}>{{2}^{\circ }}>{{1}^{\circ }}>Me>\]. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer50) Assertion (A) A particle of mass M at rest decay into two particles of masses \[\text{I}\] and \[\text{II}\] having non-zero velocities will have ratio of de-Broglie wavelengths unity. Reason (R) Here we cannot apply conservation of linear momentum. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer51) Assertion (A) To increase resolving power of a telescope, the aperture (a) of the objective should be large. Reason (R) Resolving power of the telescope is given by \[\text{III}\] Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer52) Assertion (A) If the frequency of the applied AC is doubled, then the power factor of a series R'L circuit decreases. Reason (R) Power factor of series R-L circuit is given by \[\theta =\frac{2R}{\sqrt{{{R}^{2}}+{{\omega }^{2}}{{L}^{2}}}}\] Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer53) Assertion (A) Above Curie temperature, a ferromagnetic material becomes paramagnetic. Reason (R) When a magnetic material is heated to very high temperature, it loses its magnetic properties. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer54)
Assertion (A) A charge moving in a circular orbit can produce electromagnetic wave. 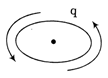 Reason (R) The source of electromagnetic wave should be in accelerated motion. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
Reason (R) The source of electromagnetic wave should be in accelerated motion. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer55)
Assertion (A) The bar magnet falling vertically along the axis of the horizontal coil will be having acceleration less than g. 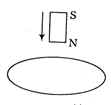 Reason (R) Clockwise current induced in the coil. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
Reason (R) Clockwise current induced in the coil. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer56) Assertion (A) The effective resistance of the network between P and Q is\[\text{IIIIII}\]. Reason (R) Symmetry can be applied to the network with respect to centre. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer57) Assertion (A) A spherical equipotential surface is not possible for a point charge. Reason (R) A spherical equipotential surface is possible inside a spherical capacitor. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer58)
Assertion (A) A wire bent into an irregular shape with the points P and Q fixed. If a current I passed through the wire, then the area enclosed by the irregular portion of the wire increases. 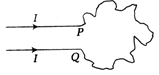 Reason (R) Opposite currents carrying wires repel each other. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
Reason (R) Opposite currents carrying wires repel each other. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer59) Assertion (A) A charge q is placed on a height \[\text{IIIIII}\] above the centre of a square of side b. The flux associated with the square is independent of side length. Reason (R) Gauss's law is independent of size of the Gaussian surface. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer60) Assertion (A) Audio signal of frequency 10 kHz cannot be transmitted over long distance without modulation. Reason (R) Length of the antenna required \[\text{IIIIII}\], should have practical value. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer61)
Following table shows the successive molar ionisation energy \[{{S}_{2}}(g)+2{{O}_{2}}(g)\to 2S{{O}_{2}}(g);\Delta G=-544KJ\] of five elements A to E. | Element | Lonisation energy \[2Zn(s)+{{S}_{2}}(g)\to 2ZnS(s);\Delta G=-293KJ\] |
| 1st | 2nd | 3rd | 4th |
| A B C D E | 2080 4600 1500 1800 3100 | 4000 500 740 580 420 | 6100 6900 7700 2700 4400 | 9400 9500 10500 11600 5900. |
| | | | | |
Which two elements are most likely to be in the same group of the periodic table?
A)
C and D
done
clear
B)
D and E
done
clear
C)
B and D
done
clear
D)
B and E
done
clear
View Answer play_arrow
-
A)
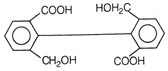
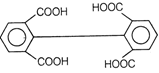
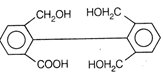
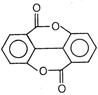
II and III are c/s and trans-isomers respectively
done
clear
B)
III and IV are trans and c/'s-isomers respectively
done
clear
C)
I and II are enantiomers
done
clear
D)
All are identical
done
clear
View Answer play_arrow
-
question_answer63) Which of the following volume (V) - temperature (T) plots represents the behaviour of one mole of an ideal gas at one atmospheric pressure?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
A)
\[BrC{{H}_{2}}-C{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}-CHBr-C{{H}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}-C{{H}_{2}}-C{{H}_{2}}Br\]
done
clear
D)
\[Br-C{{H}_{2}}-C{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer65) \[{{C}_{2}}{{H}_{5}}-C{{H}_{2}}-C{{H}_{2}}Br\]is a complex formed during the brown ring test for \[{{C}_{2}}{{H}_{5}}-CHBr-C{{H}_{3}}\] ion. In this f3 complex,
A)
there are three unpaired electrons so that its magnetic moment is 3.87 BM
done
clear
B)
NO transfer its electron to Fe24- so that iron as \[{{C}_{2}}{{H}_{5}}CHBr-C{{H}_{3}}\,\,and\,\,{{C}_{2}}{{H}_{5}}-C{{H}_{2}}-C{{H}_{2}}Br\]
done
clear
C)
the colour is because of charge transfer
done
clear
D)
All of the above statements are correct
done
clear
View Answer play_arrow
-
question_answer66) An organic compound X having molecular. mass 60 is found to contain \[PC{{l}_{5}}\]and \[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\] while rest is oxygen. On heating it gives ammonia along with a solid residue. The solid residue -2 violet colour with alkaline copper suIphate solution. The compound \[PC{{l}_{5}}\] is
A)
\[\frac{X}{a}={{\left( \frac{{{K}_{p}}}{P} \right)}^{1/2}}\]
done
clear
B)
\[\frac{X}{a}=\frac{{{K}_{p}}}{{{K}_{p}}+p}\]
done
clear
C)
\[\frac{X}{a}={{\left( \frac{{{K}_{p}}}{{{K}_{p}}+p} \right)}^{1/2}}\]
done
clear
D)
\[\frac{X}{a}={{\left( \frac{{{K}_{p}}+p}{{{K}_{p}}} \right)}^{1/2}}\]
done
clear
View Answer play_arrow
-
question_answer67)
Point out incorrect sawhorse drawing(s) for the following compound. 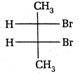
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer68) \[HN{{O}_{3}}\](potassium superoxide) is used in oxygen cylinders in space and submarines because of it
A)
absorbs \[\Delta \text{G}{}^\circ \]
done
clear
B)
produces ozone
done
clear
C)
eliminates moisture
done
clear
D)
absorbs \[C+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}Co\] and increases \[CO+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}\] content
done
clear
View Answer play_arrow
-
question_answer69) The order of reactivity of halides towards \[2Ag+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}Ag{{O}_{2}}\] mechanism is
A)
\[2Mg+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}MgO\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[\text{K},{{\text{H}}_{\text{2}}},\text{ KOH}.\text{A}l\]
done
clear
D)
\[\text{Na},{{\text{H}}_{\text{2}}},\text{ NaOH},\text{Zn}\]
done
clear
View Answer play_arrow
-
question_answer70) Arrange the given set of compounds in order of increasing boiling points. \[\text{Ca}{{\text{C}}_{\text{2}}},{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}},\text{Ca}{{\left( \text{OH} \right)}_{\text{2}}},\text{ Fe}\]1-chloropropane \[Ca,{{H}_{2}},Ca{{(OH)}_{2}},Sn\]1 so-propyl chloride \[{{V}_{c}}\]1-chlorobutane
A)
\[{{V}_{s}}\]
done
clear
B)
\[\frac{{{V}_{c}}}{{{V}_{s}}}\approx {{10}^{-3}}\]
done
clear
C)
\[\frac{{{V}_{c}}}{{{V}_{s}}}\approx {{10}^{3}}\]
done
clear
D)
\[\frac{{{V}_{c}}}{{{V}_{s}}}\approx 10\]
done
clear
View Answer play_arrow
-
question_answer71) The factor of \[\Delta G\]values is important in metallurgy. The \[\Delta G\] values for the following reactions at 800°C are given as \[{{S}_{2}}(g)+2{{O}_{2}}(g)\xrightarrow{{}}2S{{O}_{2}}(g);\]\[\Delta G=-544kJ\] \[2Zn(s)+{{s}_{2}}(g)\xrightarrow[{}]{{}}2ZnS(s);\Delta G=-293kJ\] \[2Zn(s)+{{O}_{2}}(g)\xrightarrow{{}}2ZnO(s);\Delta G=-480kJ\] The \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\] for the reaction, \[2ZnS(g)+3{{O}_{2}}(g)\xrightarrow{{}}2ZnO(s);\Delta G=-480kJ\]will be
A)
\[-731kJ\]
done
clear
B)
\[-787kJ\]
done
clear
C)
\[-534\text{ }kJ\]
done
clear
D)
\[-554\text{ }kJ\]
done
clear
View Answer play_arrow
-
question_answer72) The shapes of\[S{{F}_{4}}\]and\[Xe{{F}_{2}}\]respectively are
A)
trigonal bipyramidal and trigonai bipyramidai
done
clear
B)
see-saw and linear
done
clear
C)
T-shape and linear
done
clear
D)
square planar and trigonal bipyramidal
done
clear
View Answer play_arrow
-
question_answer73) \[{{\left( {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \right)}_{\text{2}}}\text{NH}>{{\text{C}}_{6}}{{\text{H}}_{\text{5}}}\text{NHC}{{\text{H}}_{\text{3}}}>{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}}>{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}}\] \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}}>{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{NHC}{{\text{H}}_{\text{3}}}>{{\left( {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \right)}_{\text{2}}}\text{NH}>{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}}\]X and V respectively are
A)
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}}>{{\left( {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \right)}_{\text{2}}}\text{ NH }>{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{NHC}{{\text{H}}_{\text{3}}}>{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}}\]and \[{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]
done
clear
B)
\[\text{2}.\text{4 }\times \text{l}{{0}^{-\text{4}}}\] and\[\text{mol }{{\text{L}}^{\text{-1}}}\]
done
clear
C)
\[\text{mi}{{\text{n}}^{\text{-1}}}\]and\[{{N}_{2}}{{O}_{5}}\xrightarrow{{}}2N{{O}_{2}}+\frac{1}{2}{{O}_{2}}\]
done
clear
D)
\[\text{mol }{{\text{L}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
-
question_answer74) ?a? moles of \[\text{mi}{{\text{n}}^{\text{-1}}}\] are heated in a closed container to equilibriate \[\text{2}.\text{3}\times \text{1}{{\text{0}}^{\text{-5}}}\text{ and 1}\text{.2 }\times \text{1}{{0}^{-\text{5}}}\] at a pressure of p atm. If x moles of \[\text{3}.\text{8}\times \text{1}{{\text{0}}^{-4}}\text{ and }0.\text{6}\times \text{1}{{0}^{-\text{4}}}\text{ respectively}\] dissociate at equilibrium, then
A)
\[\text{2}.\text{4}\times \text{1}{{0}^{-\text{4}}}\text{ and 1}.\text{5}\times \text{1}{{0}^{-\text{4}}}\text{ respectively}\]
done
clear
B)
\[\text{4}.\text{8}\times \text{1}{{0}^{-\text{4}}}\text{ and 1}.\text{2}\times \text{1}{{0}^{-\text{4}}}\text{ respectively}\]
done
clear
C)
\[\underset{\begin{smallmatrix} (Greater \\ Volume) \end{smallmatrix}}{\mathop{Ice}}\,\underset{\begin{smallmatrix} (Lesser \\ Volume) \end{smallmatrix}}{\mathop{Water}}\,-XKcal\]
done
clear
D)
\[Ni(s)+2A{{g}^{+}}(0.002M)\to N{{i}^{2+}}(0.160M)+2Ag(s)\]
done
clear
View Answer play_arrow
-
question_answer75) Among the metals Fe, Zn, Pb, Ag and Pt, which do not give a metal nitrate on treatment with concentrated\[(Give\,that\,{{E}^{0}}_{cell}=1.05V)\]?
A)
Fe and Pt
done
clear
B)
Fe and Zn
done
clear
C)
Fe, Ag and Pt
done
clear
D)
Pb, Ag and Pt
done
clear
View Answer play_arrow
-
question_answer76) \[\text{12}00\text{ kJ}\,\text{mo}{{l}^{\text{-1}}}\]versus T plot in the Ellingham's diagram slopes downward for the reaction
A)
\[\text{145}0\text{ kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
B)
\[\text{86}%\text{M}{{\text{g}}^{+}}+\text{14}%\text{M}{{\text{g}}^{\text{2}}}^{+}\]
done
clear
C)
\[69%M{{g}^{+}}+31%M{{g}^{2+}}\]
done
clear
D)
\[\text{14}%\text{M}{{\text{g}}^{+}}+\text{86}%\text{M}{{\text{g}}^{\text{2+}}}\]
done
clear
View Answer play_arrow
-
question_answer77) When a substance 'A' reacts with water, it produces a combustible gas 'B' and a solution of substance 'C' in water, while another substance 'H reacts with solution of 'C? to produce the same gas B on warming while 'D' can produce gas 'R on reaction with dilute\[\text{31}%\text{M}{{\text{g}}^{+}}+\text{69}%\text{M}{{\text{g}}^{\text{2}+}}\]at room temperature. 'A' imparts a deep golden yellow colour to a smokeless flame on Bunsen burner. Identify 'A', 'B', 'C" and 'D' respectively are
A)
\[C{{H}_{3}}CHO+N{{H}_{2}}.N{{H}_{2}}\to A\]
done
clear
B)
\[\xrightarrow{B}C{{H}_{3}}C{{H}_{3}}+{{N}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CH=NN{{H}_{2}}\,and\,{{C}_{2}}{{H}_{5}}ONa\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{N}{{\text{H}}_{\text{2}}}\text{ and}\,{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{ONa}\]
done
clear
View Answer play_arrow
-
question_answer78) The volume of a colloidal particle, \[C{{H}_{3}}-NH-NH-C{{H}_{3}}\,and\,{{C}_{2}}{{H}_{5}}OH\] as compared to volume of solute particle \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{N}{{\text{H}}_{\text{2}}}\text{ and }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH}\] in a true solution could be
A)
\[N{{a}^{+}}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[{{\tan }^{-1}}\left[ \frac{{{p}^{2}}+PQ+{{Q}^{2}}}{PQ} \right]\]
done
clear
D)
\[{{\tan }^{-1}}\left[ \frac{{{p}^{2}}+{{Q}^{2}}-PQ}{PQ} \right]\]
done
clear
View Answer play_arrow
-
question_answer79) Point out the correct decreasing order of \[{{\tan }^{-1}}\left[ \frac{{{p}^{2}}+{{Q}^{2}}}{2PQ} \right]\] values of following amines \[{{\sin }^{-1}}\left[ \frac{{{p}^{2}}+{{Q}^{2}}+PQ}{2PQ} \right]\] And \[\text{XX}'\]
A)
\[\text{A}=\text{1}0\text{m},\text{L}=\text{2m}\,\,\text{and}\,\,\text{d}=\text{3}0{}^\circ .\]
done
clear
B)
\[{{\mu }_{s}}=5,{{\mu }_{x}}=0.4,g=10m/{{s}^{2}}\]
done
clear
C)
\[\text{1 m}/{{\text{s}}^{\text{2}}}\]
done
clear
D)
\[\text{2 m}/{{\text{s}}^{\text{2}}}\]
done
clear
View Answer play_arrow
-
question_answer80) If the rate of decomposition of \[\text{2}.\text{4 m}/{{\text{s}}^{\text{2}}}\]during a certain time internal is \[\text{4}\times {{\text{1}}^{\text{-4}}}\text{ Wb}\] \[0.0\text{4Wb}\]\[\text{4}\mu \text{Wb}\]. \[\text{4}0\text{ mWb}\]What is the rate of formation of \[NO{{ & }_{2}}\]and \[O{{ & }_{2}}\]\[\frac{1}{4}\]\[i\]?
A)
\[\frac{2{{\mu }_{0}}i}{3\pi R}\]respectively
done
clear
B)
\[\frac{5{{\mu }_{0}}i}{12R}\]
done
clear
C)
\[\frac{6{{\mu }_{0}}i}{11R}\]
done
clear
D)
\[\frac{3{{\mu }_{0}}i}{7R}\]
done
clear
View Answer play_arrow
-
question_answer81) Consider the reaction equilibrium\[\text{P }+\text{ }\overline{\text{P}}\text{Q}\] The favourable conditions for forward reaction are
A)
low temperature, high pressure and excess of ice
done
clear
B)
low temperature, low pressure and excess of ice
done
clear
C)
high temperature, low pressure and excess of ice
done
clear
D)
high temperature, high pressure and excess of ice
done
clear
View Answer play_arrow
-
question_answer82) Calculate the emf of the cell in which of the following reaction takes place \[{{R}_{1}}\]\[{{R}_{2}}\]
A)
0.73 V
done
clear
B)
0.91 V
done
clear
C)
0.62 V
done
clear
D)
0.34 V
done
clear
View Answer play_arrow
-
question_answer83) Point out of the true statement.
A)
Photochemical smog occurs in a day time while the classical smog occur in the morning hours
done
clear
B)
Classical smog has an oxidising character whereas the photochemical smog is reducing in character
done
clear
C)
During formation of smog, the level of ozone in the atmosphere goes down
done
clear
D)
Classical smog is good for health but not photochemical smog
done
clear
View Answer play_arrow
-
question_answer84) One mole of magnesium in the vapour state absorbed \[\frac{{{R}_{1}}}{{{R}_{2}}}\] energy. If the first and second ionisation energies of Mg are 750 and \[\left| \frac{dL}{dt} \right|\] respectively, the final composition of the mixture is
A)
\[2\,\,m\,\,{{l}^{3}}{{\omega }^{2}}\sin \theta .\cos \theta \]
done
clear
B)
\[\,m\,\,{{l}^{2}}{{\omega }^{2}}\sin 2\theta \]
done
clear
C)
\[\,m\,\,{{l}^{2}}\sin 2\theta \]
done
clear
D)
\[\,{{m}^{1/2}}\,\,{{l}^{1/2}}\,\omega \,\,\sin \theta .\cos \theta \]
done
clear
View Answer play_arrow
-
question_answer85) In the following reaction\[C{{H}_{3}}CHO+N{{H}_{2}}.N{{H}_{2}}\xrightarrow{{}}A\] \[{{\mu }_{p}}\] Identify A and B.
A)
\[{{\mu }_{i}}\]
done
clear
B)
\[{{n}_{i}}\sqrt{\frac{{{\mu }_{n}}}{{{\mu }_{p}}}}\]
done
clear
C)
\[{{n}_{h}}\sqrt{\frac{{{\mu }_{n}}}{{{\mu }_{p}}}}\]
done
clear
D)
\[{{n}_{i}}\sqrt{\frac{{{\mu }_{p}}}{{{\mu }_{n}}}}\]
done
clear
View Answer play_arrow
-
question_answer86) If the distance between \[{{n}_{h}}\sqrt{\frac{{{\mu }_{p}}}{{{\mu }_{n}}}}\] and \[\left[ \text{ML}{{\text{Q}}^{-\text{2}}} \right]\] ions in sodium chloride crystal is y pm, the length of the edge of the unit cell is
A)
4y pm
done
clear
B)
y/4 pm
done
clear
C)
y/2 pm
done
clear
D)
2y pm
done
clear
View Answer play_arrow
-
question_answer87) \[[L\,\,{{T}^{-1}}{{Q}^{-1}}]\]What is the major product P in the above reaction?
A)
\[[M{{L}^{2}}\,\,{{T}^{-1}}{{Q}^{-2}}]\]
done
clear
B)
\[\left[ \text{LT}{{\text{Q}}^{-\text{1}}} \right]\]
done
clear
C)
\[{{O}_{1}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer88) Carbon and oxygen forms two compounds. Carbon content in one of them is 42.9% while in the other is 27.3%. The give data is in support with
A)
law of definite proportions
done
clear
B)
law of reciprocal proportions
done
clear
C)
law of multiple proportions
done
clear
D)
law of conservation of mass
done
clear
View Answer play_arrow
-
question_answer89)
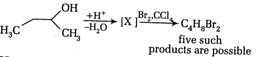 How many structures of X is possible?
How many structures of X is possible?
A)
4
done
clear
B)
5
done
clear
C)
6
done
clear
D)
3
done
clear
View Answer play_arrow
-
question_answer90) 100 mL of liquid A was mixed with 25 mL of liquid B, to give non-ideal solution of A-B. The volume of this mixture will be
A)
75 mL
done
clear
B)
125 mL exact
done
clear
C)
fluctuating between 75 mL to 125 mL
done
clear
D)
close to 125 mL but not exceed than125 mL
done
clear
View Answer play_arrow
-
question_answer91)
 Major product 'X' is
Major product 'X' is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer92) Salts of metals X, Y and Z are electrolysed under identical conditions using same quantity of electricity. It was observed that 4.2 g of X, 5.4 g of Y and 19.2 g of Z were deposited at respective cathode. If the atomic weights of X, Y, Z are 7, 27 and 64 respectively, then their ratio of valencies is
A)
1 : 2 : 3
done
clear
B)
1 : 3 : 2
done
clear
C)
2:3: 1
done
clear
D)
3:2:2
done
clear
View Answer play_arrow
-
question_answer93) Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in the presence of dilute\[HCl\]. The compound so formed is converted into tetrafluoroborate which is subsequently heated dry. The final product is
A)
2, 4, 6-tribromofluorobenzene
done
clear
B)
1, 3. 5-tribromobenzene
done
clear
C)
p-bromoaniline
done
clear
D)
o-bromofluorobenzene
done
clear
View Answer play_arrow
-
question_answer94) Three spheres of the first layer and three of the second layer enclose a site at the centre in a closest packing arrangement, this site is called
A)
interstitial void
done
clear
B)
tetrahedral void
done
clear
C)
octahedral void
done
clear
D)
cubic void
done
clear
View Answer play_arrow
-
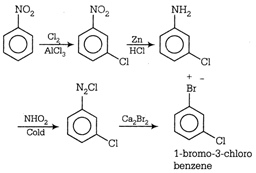
question_answer95) Which of the following is the best method for synthesis of l-bromo-3-chlorobenzene?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer96) Formation of polyethylene from calcium carbide takes place is follows: \[Ca{{C}_{2}}+2{{H}_{2}}O\xrightarrow{{}}Ca{{(OH)}_{2}}+{{C}_{2}}{{H}_{2}}\] \[{{C}_{2}}{{H}_{2}}+{{H}_{2}}\xrightarrow{{}}{{C}_{2}}{{H}_{4}}\] \[n{{C}_{2}}{{H}_{4}}\xrightarrow{{}}\,\,\,{{(-C{{H}_{2}}-C{{H}_{2}}-)}_{n}}\]. The amount of polyethylene obtained from 64.0 kg of \[5\mu A\]is
A)
27 kg
done
clear
B)
24kg
done
clear
C)
22 kg
done
clear
D)
28 kg
done
clear
View Answer play_arrow
-
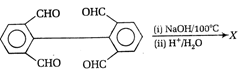
question_answer97)
Identify the product A in the given reaction,
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer98) The degeneracy of hydrogen atom that has energy equal to \[40\mu A\] is (where\[50\mu A\] = Rydberg constant)
A)
6
done
clear
B)
8
done
clear
C)
5
done
clear
D)
9
done
clear
View Answer play_arrow
-
question_answer99) Consider the statements. \[114\mu A\]Bond length in\[{{l}_{1}}\]is \[{{T}_{1}}\] greater than in\[{{l}_{2}}\]. \[{{T}_{2}}\]. Bond length in \[\frac{{{l}_{1}}+{{l}_{2}}}{2}\]is \[\sqrt{{{l}_{1}}+{{l}_{2}}}\] less than in NO. \[\frac{{{l}_{1}}{{T}_{2}}-{{l}_{2}}{{T}_{1}}}{{{T}_{2}}-{{T}_{1}}}\]. \[\frac{{{l}_{1}}{{T}_{2}}+{{l}_{2}}{{T}_{1}}}{{{T}_{1}}+{{T}_{2}}}\] has shorter bond length than \[\frac{v\,dv}{dX}=-{{\omega }^{2}}\text{x with the initial condition V}={{\text{V}}_{0}}\text{ at}\]. Which of the following statements are true?
A)
I and II
done
clear
B)
II and III
done
clear
C)
I, II and III
done
clear
D)
I and III
done
clear
View Answer play_arrow
-
question_answer100) In the following reaction, B is\[V=\sqrt{v_{0}^{2}+{{\omega }^{2}}{{X}^{2}}}\]sym-tribromobenzene
A)
salicylic acid
done
clear
B)
benzoic acid
done
clear
C)
phenol
done
clear
D)
2, 4, 6-tribromoaniline
done
clear
View Answer play_arrow
-
question_answer101) Assertion (A) Both Frenkel and Schottky defects are stoichiometric defects. Reason (R) Both defects change the density of the crystalline solid. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer102) Assertion (A) In any transition series, the magnetic moment of \[V=\sqrt{v_{0}^{2}-{{\omega }^{2}}{{X}^{2}}}\] ion first increases and then decreases. Reason (R) In any transition series, the number of unpaired electrons first increases, afterwards decrease. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer103) Assertion (A) Benzaldehyde is less reactive in comparison to ethanol towards nucleophilic attack. Reason (R) All the carbon atoms of benzaldehyde are \[V=\sqrt[3]{v_{0}^{3}+{{\omega }^{3}}{{X}^{3}}}\]hybridised. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer104) Assertion (A) Osmotic pressure of 0.1N urea solution is less than that of 0.1M\[V={{v}_{0}}-{{({{\omega }^{3}}{{X}^{3}}{{e}^{{{X}^{3}}}})}^{1/3}}\]solution. Reason (R) Osmotic pressure is not a colligative property. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer105) Assertion (A) Gabriel phthalimide reaction can be used to prepare aryl and alkyl amines. Reason (R) Aryl halides have same reactivity as alkyl halides towards nucleophilic substitution reactions. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer106) Assertion (A) Iron is protected from corrosion by connecting magnesium metal with it. Reason (R) Iron acts as cathode and magnesium as anode which gradually disappears. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer107) Assertion (A) Elementary phosphorus exists in three principal allotropic forms, i.e. white (or yellow), red (or violet) and black. Reason (R) of the three forms, white phosphorus is the most important and most reactive. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer108) Assertion (A) Chlorine reacts more rapidlywith \[\frac{2\,{{m}^{2}}}{3}g\] in comparision to \[\frac{4\,{{m}^{2}}}{3}g\]. Reason (R) \[\frac{\,{{m}^{2}}}{\sqrt{2}}g\]bond is stronger in comparison to \[5\Omega \] bond. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer109) Assertion (A) A solution of sucrose in water is dextrorotatory while on hydrolysis in presence of little hydrochloric acid, it becomes laevorotatory. Reason (R) Sucrose on hydrolysis gives unequal amounts of glucose and fructose as a result of which change in sign of rotation is observed. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer110) Assertion (A) tert-butyl methyl ether on treatment with HI at 100°C gives a mixture of methyl iodide and tert-butyl alcohol. Reason (R) This reaction occur via \[2A\] mechanism. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer111) Assertion (A) In comparison to ethyl chloride, it is not easy to carry out nucleophilic substitution on vinyl chloride. Reason (R) Vinyl group is an electron donating. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer112) Assertion (A) Ranitidine is used to treat hyperacidity and brompheniramine is used to treat hypersensitivity. Reason (R) Both of these drugs are antihistamines. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer113) Assertion (A) Graphite is a good conductor of heat and electricity. Reason (R) Graphite has all the electrons firmly held together in C?C a-bonds. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer114) Assertion (A) Sodium reacts with oxygen to form \[\frac{12}{7}A\] but potassium reacts with oxygen to form\[1A\]. Reason (R) Potassium is more reactive metal than sodium. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer115) Assertion (A) A mixture of o-nitrophenol and p-nitrophenol can be separated by steam distillation. Reason (R) p-nitrophenol is steam volatile whereas o-nitrophenol is not steam volatile. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer116) Assertion (A) Friedel-Crafts reaction of benzene with n-propyi chloride on heating produce isopropyi benzene. Reason (R) Benzene undergoes electrophilic substitution easily. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer117) Assertion (A) Presence of green plant is essential for greenhouse effect. Reason (R) Chlorophyll of green plants causes greenhouse effect. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer118) Assertion (A) Neoprene can be further hardened by heating with the sulphur. Reason (R) It contains allylic double bond which help in introducing sulphur bridges between different polymer chains. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer119) Assertion (A) \[{{H}_{2}}O\] is the only hydride of group-16 which is liquid at ordinary temperature. Reason (R) In ice, each oxygen atom is surrounded by two covalent bonds and two hydrogen bonding. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer120) Assertion (A) Carbonate and hydroxide ores are concentrated by froth floatation process. Reason (R) In froth floatation process, mineral oil is used due to it preferentially wets the gangue particles. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer121) The antibiotics have no effect on viruses because
A)
viruses show metabolism of their own
done
clear
B)
viruses are too small in size for antibiotics to act upon them
done
clear
C)
viruses show no metabolism of their own
done
clear
D)
viruses produce a thick covering and encyst themselves as endospores
done
clear
View Answer play_arrow
-
question_answer122) Schizocoelomates and enterocoelomates are
A)
acoelomates
done
clear
B)
invertebrates
done
clear
C)
true coelomates
done
clear
D)
echinoderms only
done
clear
View Answer play_arrow
-
question_answer123) Cestoda are distinguished from other flatworms by the absence of
A)
digestive system
done
clear
B)
nervous system
done
clear
C)
excretory system
done
clear
D)
reproductive system
done
clear
View Answer play_arrow
-
question_answer124) Which one of the following is referred as 'living fossils'?
A)
Sarcoptes
done
clear
B)
Daphnia
done
clear
C)
Limulus
done
clear
D)
Balanus
done
clear
View Answer play_arrow
-
question_answer125)
Match the following columns. | Column I | Column II |
| A. Sea lemon B. Sea mussel C. Garden snail D. Grey slug | 1. Doris 2. Mytilus 3. Helix 4. Umax |
A)
Codes A B C D 1 2 3 4
done
clear
B)
4 3 2 1
done
clear
C)
4 2 3 1
done
clear
D)
3 4 1 2
done
clear
View Answer play_arrow
-
question_answer126) The small projections present on the surface of pineapple fruit represent
A)
persistent styles
done
clear
B)
persistent stamens
done
clear
C)
persistent calyx
done
clear
D)
persistent bracts
done
clear
View Answer play_arrow
-
question_answer127) Pith cavity occurs in the stem of
A)
Helianthus
done
clear
B)
Zea mays
done
clear
C)
Cucurbita
done
clear
D)
Dracaena
done
clear
View Answer play_arrow
-
question_answer128) Pollination in Rafflesia is occurred by
A)
carrion flies
done
clear
B)
elephant
done
clear
C)
bat
done
clear
D)
crow
done
clear
View Answer play_arrow
-
question_answer129) Which of the following statement is true?
A)
Vessels are unicellular and with narrow lumen
done
clear
B)
Vessels are multicellular and with wide lumen
done
clear
C)
Tracheids are unicellular and with wide lumen
done
clear
D)
Tracheids are multicellular and with narrow lumen
done
clear
View Answer play_arrow
-
question_answer130) Haversian canal in the bone of mammals are connected by small blood vessel canal called
A)
Schlemm's canal
done
clear
B)
Volkmann's canal
done
clear
C)
Portal capillaries
done
clear
D)
Sinuses
done
clear
View Answer play_arrow
-
question_answer131) If a biochemical analysis of mitochondria has to be done, the best procedure would be
A)
select cells which have a larger number of mitochondria
done
clear
B)
plasmolyse the cell and filter out the mixture and take the debris
done
clear
C)
grind the cells and filter out the mixture and take the debris
done
clear
D)
subject the cell to cell fractionation (centrifuge) and obtain mitochondria
done
clear
View Answer play_arrow
-
question_answer132) Choose the correct statement.
A)
All proteins have 20 amino acids
done
clear
B)
Both ends of a protein are similar
done
clear
C)
All proteins are soluble
done
clear
D)
Proteins are formed by peptide bonds
done
clear
View Answer play_arrow
-
question_answer133)
Match the following column I with column II. | Column I | Column II |
| A. Agrostology B. Smallest flowering Plant C. Tectonic D. Ecesis E. Euphanix | 1. Earth 2.Genetic engineering 3. Migration 4. Wolffia 5. Grass |
A)
Codes A B C D E 5 4 1 3 2
done
clear
B)
1 2 3 4 5
done
clear
C)
2 3 1 5 4
done
clear
D)
5 4 3 2 1 4 5 2 1 3
done
clear
View Answer play_arrow
-
question_answer134) What is true of urea biosynthesis?
A)
Uric acid is starting point
done
clear
B)
Urea is synthesised in lysosomes
done
clear
C)
Urea cycle enzymes are located inside mitochondria
done
clear
D)
Urea is synthesised in kidney
done
clear
View Answer play_arrow
-
question_answer135) In old age, stiffness of joints is due to the
A)
hardening of bones
done
clear
B)
inefficiency of muscles
done
clear
C)
decrease in synovial fluid
done
clear
D)
enlargement of bones
done
clear
View Answer play_arrow
-
question_answer136) Node of Ranvier occurs where
A)
nerve is covered with myelin sheath
done
clear
B)
neurilemma is discontinuous
done
clear
C)
neurilemma and myelin sheath are discontinuous
done
clear
D)
myelin sheath is discontinuous
done
clear
View Answer play_arrow
-
question_answer137) Correct hormonal sequence in the case of 'menstruation as
A)
oestrogen, FSH, progesterone
done
clear
B)
oestrogen, progesterone, FSH
done
clear
C)
FSH, progesterone, oestrogen
done
clear
D)
FSH, oestrogen, progesterone
done
clear
View Answer play_arrow
-
question_answer138) In blue-green algae, photosystem-II contains important pigment concerned with photolysis of water. It is a
A)
phycocyanin
done
clear
B)
cytochrome-c
done
clear
C)
chlorophyll-b
done
clear
D)
\[\omega =\sqrt{rg\,\sin \theta }\]
done
clear
View Answer play_arrow
-
question_answer139)
Identify the process taking place in this experiment. 
A)
Ringing experiment for translocation of sap
done
clear
B)
Demonstration of root pressure
done
clear
C)
Eosin test to demonstrate ascent of sap
done
clear
D)
Demonstration of transpiration
done
clear
View Answer play_arrow
-
question_answer140) Which of the following statement is incorrect regarding the band region of polytene chromosome?
A)
Feulgen negative area
done
clear
B)
Absorb ultraviolet light at 2600 A
done
clear
C)
Chromonemata is tightly packed
done
clear
D)
Stain intensily with basic stain
done
clear
View Answer play_arrow
-
question_answer141) Refrigerated fruits maintain flavour and taste for longer period due to
A)
non-availability of \[\omega =\sqrt{g/r\,\,\cos \theta }\]
done
clear
B)
presence of excess of \[\omega =\sqrt{\frac{gr}{\cos \theta }\,\,}\]
done
clear
C)
presence of excess humidity
done
clear
D)
slower rate of respiration
done
clear
View Answer play_arrow
-
question_answer142) The biological clock measures the length at each night by the
A)
relative amount of red absorbing and far-red absorbing phytochrome present at dawn
done
clear
B)
amount of far-red absorbing phytochrome at dusk
done
clear
C)
relative amount of red absorbing and far red absorbing phytochrome at mid day
done
clear
D)
rate at which are kind of phytochrome is converted to the other
done
clear
View Answer play_arrow
-
question_answer143) In the homeostatic control of blood sugar level, which organs function as modulator and effector respectively?
A)
Liver and islet of Langerhans
done
clear
B)
Hypothalamus and liver
done
clear
C)
Hypothalamus and islet of Langerhans
done
clear
D)
Islet of Langerhans and hypothalamus
done
clear
View Answer play_arrow
-
question_answer144) A colour blind man marry with a daughter of colour blind father, the generation will be
A)
there will be no daughter colour blind
done
clear
B)
all sons will be colour blind
done
clear
C)
all daughter will be colour blind
done
clear
D)
half sons will be colour blind
done
clear
View Answer play_arrow
-
question_answer145) Which one of the following organisms is correctly matched with its three characteristics?
A)
Pea- \[\omega =\sqrt{\frac{gr}{\tan \theta }\,\,}\]-pathway, endospermic seed, veaxillary aestivation
done
clear
B)
Tomato- twisted aestivation, axile placentation, berry
done
clear
C)
Onion- Bulb, imbricate aestivation, axile placentation
done
clear
D)
Maize- \[\pi /2\]-pathway, closed vascular bundle, scutellum
done
clear
View Answer play_arrow
-
question_answer146) The domestic sewage in large cities
A)
has a high BOD as it contains both aerobic and bacteria
done
clear
B)
is processed by aerobic and then anaerobic bacteria in the secondary treatment in Sewage Treatment Plants (STPs)
done
clear
C)
when treated in STPs does not really require the aeration step as the sewage contains adequate oxygen
done
clear
D)
has very high amounts of suspended solids and dissolved salts
done
clear
View Answer play_arrow
-
question_answer147) The first clinical gene therapy was given for treating
A)
diabetes mellitus
done
clear
B)
chickenpox
done
clear
C)
rheumatoid arthritis
done
clear
D)
adenosine deaminase deficiency
done
clear
View Answer play_arrow
-
question_answer148) Which one of the following human organs is called the 'graveyard of RBCs?
A)
Gall bladder
done
clear
B)
Kidney
done
clear
C)
Spleen
done
clear
D)
Liver
done
clear
View Answer play_arrow
-
question_answer149) Biolistic (gene gun) is suitable for
A)
disarming pathogen vectors
done
clear
B)
transformation of plant ceil
done
clear
C)
constructing recombinant DNA by joining with vectors
done
clear
D)
DNA fingerprinting
done
clear
View Answer play_arrow
-
question_answer150) Which one of the following generally act as an antagonists to gibberellins?
A)
Zeatin
done
clear
B)
Ethylene
done
clear
C)
ABA
done
clear
D)
!AA
done
clear
View Answer play_arrow
-
question_answer151) Select the correct statement about biodiversity.
A)
The desert area of Rajasthan and Gujarat have a very high level of desert animals .
done
clear
B)
Large scale planting of Bt cotton has no adverse effect on biodiversity.
done
clear
C)
Western Gnats have a very high degree of species richness and endemism.
done
clear
D)
Conservation of biodiversity is just a fad pursued by the developed countries.
done
clear
View Answer play_arrow
-
question_answer152) Which one of the following structure is an organelle within an organelle?
A)
Ribosome
done
clear
B)
Peroxysome
done
clear
C)
ER
done
clear
D)
Mesosome
done
clear
View Answer play_arrow
-
question_answer153) Which of the following is the relatively most accurate method for dating fossils?
A)
Potassium- argon method
done
clear
B)
Uranium-lead method
done
clear
C)
Electron spin resonance method
done
clear
D)
Radio carbon method
done
clear
View Answer play_arrow
-
question_answer154) The 24-hour (diurnal) rhythm of our body such as the sleep-wake cycle is regulated by the hormone
A)
calcitonin
done
clear
B)
proiactin
done
clear
C)
adrenaline
done
clear
D)
melatomn
done
clear
View Answer play_arrow
-
question_answer155) In Kranz anatomy, the bundle sheath cells have
A)
thin walls, many intercellular space and no chloroplast
done
clear
B)
thick walls, no intercellular spaces and large number of chloroplasts
done
clear
C)
thin walls, no intercellular spaces and several chloroplasts
done
clear
D)
thick walls, many intercellular space and few chloroplasts
done
clear
View Answer play_arrow
-
question_answer156) Which one of the following is essential for photolysis of water?
A)
Manganese
done
clear
B)
Zinc
done
clear
C)
Copper
done
clear
D)
Boron
done
clear
View Answer play_arrow
-
question_answer157) In a plant, red fruit (R) dominant over yellow fruit (r) and tallness (T) is dominant over shortness (t). If a plant with RRTT genotype is crossed with a plant that is rrtt. Then
A)
25% will be tall with red fruit
done
clear
B)
50% will be tall with red fruit
done
clear
C)
75% will be tall with red fruit
done
clear
D)
All of the offspring will be tall with red fruits
done
clear
View Answer play_arrow
-
question_answer158) Which one of the following pairs is not correctly matched?
A)
Vitamin-\[\pi /3\]- Pernicious anaemia
done
clear
B)
Vitamin-\[-\pi /6\]-Loss of appetite
done
clear
C)
Vitamin-\[\pi \]- Beri-beri .
done
clear
D)
Vitamin-\[250\mu F\]- Pellagra
done
clear
View Answer play_arrow
-
question_answer159) What would happen, if in a gene encoding a polypeptide of 50 amino acids, 25thcodon (UAU) is mutated to UAA?
A)
A polypeptide of 49 amino acids will be formed
done
clear
B)
A polypeptide of 25 amino acids will be formed
done
clear
C)
A polypeptide of 24 amino acids will be formed
done
clear
D)
Two polypeptides of 24 and 25 amino acids will be formed
done
clear
View Answer play_arrow
-
question_answer160) Shprt-lived immunity acquired from mother to foetus placenta or through mother's milk to the infant is categorised as
A)
cellular immunity
done
clear
B)
innate non-specific immunity
done
clear
C)
active immunity
done
clear
D)
passive immunity
done
clear
View Answer play_arrow
-
question_answer161) Assertion (A) Coacervates are believed to 16 be the precursors of life. Reason (R) Coacervates were self-duplicat ing aggregates of proteins surrounded by lipid molecules. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer162) Assertion (A) Genecology is the study of genetic compositions and changes is relation to the origin of ecades, ecotypes, new sps., etc. Reason (R) Autecology deals with the study of a group of organisms. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer163) Assertion (A) UV-radiation causes photodissociation of enzyme into 02 and 0. Thus causing damage to the stratospheric ozone layer. Reason (R) Ozone hole is resulting in global 1 warming and climate change. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer164) Assertion (A) A morphology based approach to taxonomy is called alpha taxonomy and it is old fashioned. Reason (R) A multidisciplmary approach to taxonomy called omega taxonomy is favoured in recent years. As, it excludes morphological features. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer165) Assertion (A) A network of food chains existing together in an ecosystem is known as food web. Reason (R) An animal like kite cannot be apart of food web. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer166) Assertion (A) Smaller the organism, higher is the rate of metabolism per gram weight. Reason (R) The heart rate of six months old baby is much higher than that of an old person. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer167) Assertion (A) Female gametophyte in angiosperm is eight nucleate. Reason (R) Double fertilisation occurs in angiosperms. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer168) Assertion (A) Photomodulation of flowering is a phytochrome regulated process. Reason (R) Active form of phytochrome directly induces floral induction in shoot bud. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer169) Assertion (A) Cyclic pathway of photosynthesis first appeared in some eubacterial sps. Reason (R) \[20\Omega \] started accumulating in the atmospheres after the non-cyclic pathway of photosynthesis evolve. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer170) Assertion (A) Under condition of high light intensity and limited \[9\times {{10}^{4}}\,\,Hz\]supply, photorespiration has a useful role in protecting the plants from photooxidative damage. Reason (R) If enough \[16\times {{10}^{7}}\,\,Hz\] is not available to utilise light energy for carboxylation to proceed, the excess energy may not cause damage to plants. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer171) Assertion (A) A cell membrane shows fluid behaviour. Reason (R) A membrane is a mosaic or composite of diverse lipids and proteins. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer172) Assertion (A) A coenzyme or metal ion that is very tightly bound to enzyme protein called prosthetic group. Reason (R) A complete, catalytically active enzymb together with its bound prosthetic group is called apoenzyme. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer173) Assertion (A) \[8\times {{10}^{5}}\,\,Hz\]-phase is also called anaphase, as during this phase the cell stores ATP for cell division. Reason (R) Cell produces structural and functional proteins. Cell's metabolic rate is high and is controlled by the enzymes. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer174) Assertion (A) Secondary growth in dicot roots occur with the help of vascular cambium and phellogen. Reason (R) Vascular cambium is formed from conjuctive parenchyma and part of pericycle. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer175) Assertion (A) Most minerals must enter the root by active absorption into the cytoplasm of epidermal cells. Reason (R) This transportation needs energy in the form of ATP. Some ions also move into the epidermal cells passively. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer176) Assertion (A) The chemical potential of pure water at normal temperature and pressure is zero. Reason (R) In solution, value of water potential is always positive. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer177) Assertion (A) Chlorine is absorbed as \[9\times {{10}^{3}}\,\,Hz\]ions. Its precise role is not well known. However with \[\lambda \,~\text{is greater than 2d}\] and \[\lambda \,quals2d\], it help in determining solute concentration and anion-cation balance is cells. Reason (R) Chlorine plays an important role in photosynthesis and takes part in the water splitting reaction, thus releasing \[\Rightarrow \]. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer178) Assertion (A) In alcoholic drink, the alcohol is converted into glucose in liver. Reason (R) Liver cells are able to produce glucose from alcohol by back fermentation. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer179) Assertion (A) Liver is the largest gland of the body. The hepatic lobules are the structural and functional units of liver containing hepatic cells arranged in the form of cords. Reason (R) Each lobule of the liver is covered by a thin connective tissue sheath called the Glisson's capsule. The bile is secreted by hepatic cells. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow
-
question_answer180) Assertion (A) Tidal volume is the volume of air inspired or expired with the normal breath. Reason (R) Adult person contains 500 mL expired or inspired volumes of air with each normal breath. Each of these questions contains two statements. Assertion (A) and Reason (R). Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
A)
If both Assertion and Reason are true and Reason is correct explanation of Assertion.
done
clear
B)
if both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
done
clear
C)
If Assertion is true but Reason is false.
done
clear
D)
If both Assertion and Reason are false.
done
clear
View Answer play_arrow


 A voltmeter of resistance \[150\Omega \]. is connected across A and 5. The potential drop across and C measured by voltmeter is
A voltmeter of resistance \[150\Omega \]. is connected across A and 5. The potential drop across and C measured by voltmeter is

 The coefficient of friction between block A and the wedge is 2/3, while that for block B and the wedge is 1/3. If the whole system is released from rest, then acceleration of block A is
The coefficient of friction between block A and the wedge is 2/3, while that for block B and the wedge is 1/3. If the whole system is released from rest, then acceleration of block A is

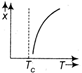
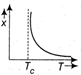
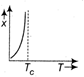
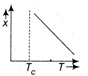



 Reason (R) The source of electromagnetic wave should be in accelerated motion. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
Reason (R) The source of electromagnetic wave should be in accelerated motion. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
 Reason (R) Clockwise current induced in the coil. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
Reason (R) Clockwise current induced in the coil. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
 Reason (R) Opposite currents carrying wires repel each other. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
Reason (R) Opposite currents carrying wires repel each other. Each of these questions contains two statements. Assertion and Reason. Each of these questions also has four alternative choices, only one of-which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
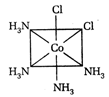
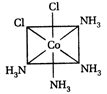 \[\Delta G\] \[2Zn(s)+3{{O}_{2}}(g)\to 2ZnO(g)+2S{{O}_{2}}(g)\]
\[\Delta G\] \[2Zn(s)+3{{O}_{2}}(g)\to 2ZnO(g)+2S{{O}_{2}}(g)\] 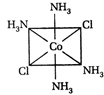
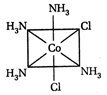 \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH}=\text{C}{{\text{H}}_{\text{2}}}\text{+HBr}\] \[\xrightarrow{\text{ROOR (peroxide)}}\underset{Major}{\mathop{(X)}}\,+\underset{Minor}{\mathop{(Y)}}\,\]Which of the following statements is false?
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH}=\text{C}{{\text{H}}_{\text{2}}}\text{+HBr}\] \[\xrightarrow{\text{ROOR (peroxide)}}\underset{Major}{\mathop{(X)}}\,+\underset{Minor}{\mathop{(Y)}}\,\]Which of the following statements is false?
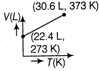
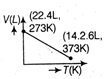
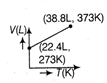
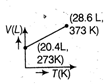
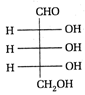
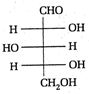 (b) (c) (d)
(b) (c) (d) 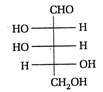
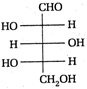

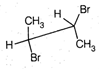
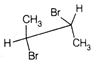
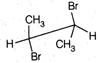
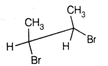
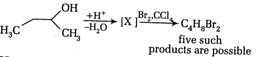 How many structures of X is possible?
How many structures of X is possible?
 Major product 'X' is
Major product 'X' is