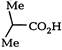
 AIEEE Solved Paper-2006
AIEEE Solved Paper-2006
A) II < IV < I < III
B) IV < I < III < II
C) IV < I < II < III
D) I < IV < III < II
Correct Answer: C
Solution :
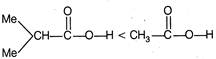
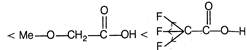 \[-l\] effect increases acidity. F \[+l\] effect decreases acidity. \[-C{{F}_{3}}\]exerting more \[-\,l\] effect than\[-\text{ }OMe\] \[M{{e}_{2}}CH-\]exerting more \[+\,l\] effect than\[C{{H}_{3}}\]
\[-l\] effect increases acidity. F \[+l\] effect decreases acidity. \[-C{{F}_{3}}\]exerting more \[-\,l\] effect than\[-\text{ }OMe\] \[M{{e}_{2}}CH-\]exerting more \[+\,l\] effect than\[C{{H}_{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
