A) \[ds{{p}^{3}}\]hybridisation
B) \[s{{p}^{3}}{{d}^{2}}\]hybrfdisation
C) \[ds{{p}^{2}}\]hybridisation
D) \[s{{p}^{3}}d\]hybridisation
Correct Answer: B
Solution :
\[s{{p}^{3}}{{d}^{2}}\]hybridisation has octahedral structure such that four hybrid orbitals are at\[90{}^\circ \]w.r.t. each other and others two at\[90{}^\circ \]with first four.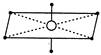
You need to login to perform this action.
You will be redirected in
3 sec
