A) \[Xe{{F}_{4}}\]
B) \[S{{F}_{4}}\]
C) \[BF_{4}^{-}\]
D) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
Correct Answer: C
Solution :
Tetrahedral structure is associated with\[s{{p}^{3}}\] hybridised central atom without any lone pair. The structure of all the compounds given are as follows| \[Xe{{F}_{4}}\] |  | Distorted octahedral |
| \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] | | Square planar |
| \[S{{F}_{4}}\] | 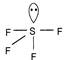 | Distorted trigonal bipyramidal |
| \[BF_{4}^{-}\] | 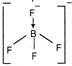 | Tetrahedral |
You need to login to perform this action.
You will be redirected in
3 sec
