A) \[{{T}_{1}}+{{T}_{2}}\]
B) \[\frac{({{T}_{1}}+{{T}_{2}})}{2}\]
C) \[\frac{{{T}_{1}}{{T}_{2}}({{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}})}{{{p}_{1}}{{V}_{1}}{{T}_{2}}+{{p}_{2}}{{V}_{2}}{{T}_{1}}}\]
D) \[\frac{{{T}_{1}}{{T}_{2}}({{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}})}{{{p}_{1}}{{V}_{1}}{{T}_{1}}+{{p}_{2}}{{V}_{2}}{{T}_{2}}}\]
Correct Answer: C
Solution :
\[{{n}_{1}}=\frac{{{p}_{1}}{{V}_{1}}}{R{{T}_{1}}}\] \[{{n}_{2}}=\frac{{{p}_{2}}{{V}_{2}}}{R{{T}_{2}}}\]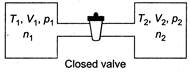 From ideal gas equation, (as P and T will become same) \[n_{1}^{'}=\frac{p{{V}_{1}}}{RT}\] \[n_{2}^{'}=\frac{p{{V}_{2}}}{RT}\]
From ideal gas equation, (as P and T will become same) \[n_{1}^{'}=\frac{p{{V}_{1}}}{RT}\] \[n_{2}^{'}=\frac{p{{V}_{2}}}{RT}\] 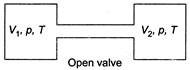 \[{{n}_{1}}+{{n}_{2}}=n_{1}^{'}+n_{2}^{'}\] (moles of gas remain same) \[\frac{1}{R}\left[ \frac{{{p}_{1}}{{V}_{1}}}{{{T}_{1}}}+\frac{{{p}_{2}}{{V}_{2}}}{{{T}_{2}}} \right]=\frac{p}{RT}({{V}_{1}}+{{V}_{2}})\] \[\Rightarrow \]\[\frac{P}{T}=\frac{{{p}_{1}}{{V}_{1}}{{T}_{2}}+{{p}_{2}}{{V}_{2}}{{T}_{1}}}{{{T}_{1}}{{T}_{2}}({{V}_{1}}+{{V}_{2}})}\] ?..(i) Internal energy of the system (air in two vessels) remains same, before and after opening of valve, so \[\frac{f{{n}_{1}}R{{T}_{1}}}{2}+\frac{f{{n}_{2}}R{{T}_{2}}}{2}=\frac{f({{n}_{1}}+{{n}_{2}})RT}{2}\] where, f is degree of freedom of gas molecules \[\left[ U=\frac{fnRT}{2} \right]\] \[\Rightarrow \] \[{{n}_{1}}{{T}_{1}}+{{n}_{2}}{{T}_{2}}=({{n}_{1}}+{{n}_{2}})T\] \[\Rightarrow \] \[\frac{{{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}}}{R}=\left( \frac{{{p}_{1}}{{V}_{1}}}{{{T}_{1}}}+\frac{{{p}_{2}}{{V}_{2}}}{{{T}_{2}}} \right)\frac{T}{R}\] \[\Rightarrow \]\[T=\frac{({{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}}){{T}_{1}}{{T}_{2}}}{{{p}_{1}}{{V}_{1}}{{T}_{2}}+{{p}_{2}}{{V}_{2}}{{T}_{1}}}\] From Eq. (i) we can find the equilibrium pressure also.
\[{{n}_{1}}+{{n}_{2}}=n_{1}^{'}+n_{2}^{'}\] (moles of gas remain same) \[\frac{1}{R}\left[ \frac{{{p}_{1}}{{V}_{1}}}{{{T}_{1}}}+\frac{{{p}_{2}}{{V}_{2}}}{{{T}_{2}}} \right]=\frac{p}{RT}({{V}_{1}}+{{V}_{2}})\] \[\Rightarrow \]\[\frac{P}{T}=\frac{{{p}_{1}}{{V}_{1}}{{T}_{2}}+{{p}_{2}}{{V}_{2}}{{T}_{1}}}{{{T}_{1}}{{T}_{2}}({{V}_{1}}+{{V}_{2}})}\] ?..(i) Internal energy of the system (air in two vessels) remains same, before and after opening of valve, so \[\frac{f{{n}_{1}}R{{T}_{1}}}{2}+\frac{f{{n}_{2}}R{{T}_{2}}}{2}=\frac{f({{n}_{1}}+{{n}_{2}})RT}{2}\] where, f is degree of freedom of gas molecules \[\left[ U=\frac{fnRT}{2} \right]\] \[\Rightarrow \] \[{{n}_{1}}{{T}_{1}}+{{n}_{2}}{{T}_{2}}=({{n}_{1}}+{{n}_{2}})T\] \[\Rightarrow \] \[\frac{{{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}}}{R}=\left( \frac{{{p}_{1}}{{V}_{1}}}{{{T}_{1}}}+\frac{{{p}_{2}}{{V}_{2}}}{{{T}_{2}}} \right)\frac{T}{R}\] \[\Rightarrow \]\[T=\frac{({{p}_{1}}{{V}_{1}}+{{p}_{2}}{{V}_{2}}){{T}_{1}}{{T}_{2}}}{{{p}_{1}}{{V}_{1}}{{T}_{2}}+{{p}_{2}}{{V}_{2}}{{T}_{1}}}\] From Eq. (i) we can find the equilibrium pressure also.
You need to login to perform this action.
You will be redirected in
3 sec
