A) \[-N{{H}_{2}},-COOH\]
B) \[-N{{H}_{2}},-S{{O}_{3}}H\]
C) Both (a) and (b)
D) None of these
Correct Answer: C
Solution :
For the formation of Zwitter ion, basic part and acidic part both should be stronger one. Zwitter ion is a neutral molecule with a positive and a negative electrical charge, though multiple positive and negative charge can be present. A substance can form Zwitter ion, if it has \[-N{{H}_{2}}\] group and \[-COOH\] group as well as if it has \[-N{{H}_{2}}\] group and \[-S{{O}_{3}}H\] group. Zwitter ion formed by \[-N{{H}_{2}}\] group and \[-COOH\] group \[{{H}_{2}}N-\overset{\begin{smallmatrix} R \\ \,\,| \end{smallmatrix}}{\mathop{C}}\,H-COOH\overset{{}}{leftrightarrows}{{H}_{3}}\overset{+}{\mathop{N}}\,-\overset{\begin{smallmatrix} R \\ | \end{smallmatrix}}{\mathop{C}}\,H-\underset{Zwitter\,\,ion}{\mathop{CO{{O}^{-}}}}\,\] Zwitter ion formed by \[-N{{H}_{2}}\] and \[-S{{O}_{3}}H\] group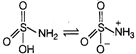
You need to login to perform this action.
You will be redirected in
3 sec
