A) \[\underline{A}\,l{{H}_{3}}\] changes to \[\underline{A}\,lH_{4}^{-}\]
B) \[{{H}_{2}}\underline{O}\] changes to \[{{H}_{3}}{{O}^{+}}\]
C) \[\underline{N}{{H}_{3}}\] changes to \[NH_{4}^{+}\]
D) in all the above cases
Correct Answer: A
Solution :
| \[Al{{H}_{3}}\] \[s{{p}^{2}}\] | 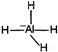 |
Change in hybridization as well as in shape | |
| \[{{H}_{2}}O\] \[s{{p}^{3}}\] \[(2\,bp+2\,lp)\] | \[{{H}_{3}}{{O}^{+}}\] \[s{{p}^{3}}\]\[(3\,bp+1\,lp)\] | Change in shape only, bent to pyramidal | |
| \[N{{H}_{3}}\] \[s{{p}^{3}}\] \[(3\,bp+1lp)\] | \[NH_{4}^{+}\] \[s{{p}^{3}}\] \[(4\,bp)\] | Change in shape only, pyramidal to tetrahedral |
You need to login to perform this action.
You will be redirected in
3 sec
