question_answer 1) In which process the pV indicator diagram is a straight line parallel to volume axis
A)
isothermal
done
clear
B)
isobaric
done
clear
C)
irreversible
done
clear
D)
adiabatic
done
clear
View Answer play_arrow
question_answer 2) A body executes simple harmonic motion under the action of force F1 with a time period \[\frac{4}{5}\] s. If die force is changed to F2 it executes simple harmonic motion with time period \[\frac{3}{5}\] s. If both forces F1 and F2 act simultaneously in the same direction on the body, its time period will be
A)
\[\frac{12}{25}\,s\]
done
clear
B)
\[\frac{24}{25}\,s\]
done
clear
C)
\[\frac{35}{24}\,s\]
done
clear
D)
\[\frac{15}{12}\,s\]
done
clear
View Answer play_arrow
question_answer 3) A diatomic gas is heated at constant pressure. What fraction of the heat energy is used to increase the internal energy?
A)
\[\frac{3}{5}\]
done
clear
B)
\[\frac{3}{7}\]
done
clear
C)
\[\frac{5}{7}\]
done
clear
D)
\[\frac{5}{9}\]
done
clear
View Answer play_arrow
question_answer 4) In interference pattern, the energy is
A)
created at the maximum
done
clear
B)
destroyed at the minimum
done
clear
C)
conserved but redistributed
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 5) A red flower kept in green light will appear
A)
red
done
clear
B)
yellow
done
clear
C)
black
done
clear
D)
white
done
clear
View Answer play_arrow
question_answer 6) A band playing music at a frequency f is moving towards a wall at a speed vb. A motorist is following the band with a speed vm. If v be the speed of the sound, the expression for beat frequency heard by motorist is
A)
\[\frac{v+{{v}_{m}}}{v+{{v}_{b}}^{2}}f\]
done
clear
B)
\[\frac{v+{{v}_{m}}}{v-{{v}_{b}}}f\]
done
clear
C)
\[\frac{2{{v}_{b}}(v+{{v}_{m}})}{{{v}^{2}}-{{v}_{b}}^{2}}f\]
done
clear
D)
\[\frac{2{{v}_{m}}(v+{{v}_{b}})}{{{v}^{2}}-{{v}_{m}}^{2}}f\]
done
clear
View Answer play_arrow
question_answer 7) An eye specialist prescribes spectacles having a combination of a convex lens of focal length 40 cm in contact with a concave lens of focal length 25 cm. The power of this lens combination will be
A)
+ 1.5 D
done
clear
B)
- 1.5 D
done
clear
C)
+ 6.67 D
done
clear
D)
- 6.67 D
done
clear
View Answer play_arrow
question_answer 8) When light wave suffers reflection at the interface between air and glass, the change of phase of reflected wave is equal to
A)
zero
done
clear
B)
n / 2
done
clear
C)
n
done
clear
D)
2 n
done
clear
View Answer play_arrow
question_answer 9) A lens behaves as a converging lens in air and diverging lens in water. The refractive index of the material of the lens is
A)
equal to that of water
done
clear
B)
less than that of water
done
clear
C)
greater than that of water
done
clear
D)
Nothing can be predicted
done
clear
View Answer play_arrow
question_answer 10) The work function of a substance is 4.0 eV. The longest wavelength of light that can cause photoelectron emission from this substance is approximately
A)
540 nm
done
clear
B)
400 nm
done
clear
C)
310 nm
done
clear
D)
220 nm
done
clear
View Answer play_arrow
question_answer 11) The electron emitted in beta radiation originates from
A)
inner orbits of atoms
done
clear
B)
free electron existing in nuclei
done
clear
C)
decay of neutron in the nucleus
done
clear
D)
phoron escaping from the nucleus
done
clear
View Answer play_arrow
question_answer 12) If elements with principal quantum number n > 4 were not allowed in nature, then the number of possible elements would be
A)
32
done
clear
B)
60
done
clear
C)
18
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 13) The magnifying power of telescope is high if
A)
both objective and eye-piece have short focal length
done
clear
B)
both objective and eye-piece have long focal length
done
clear
C)
the objective has a long focal length and the eye-piece has a short focal length
done
clear
D)
the objective has a short focal length and the eye piece has a long focal length
done
clear
View Answer play_arrow
question_answer 14) What is the current through an ideal pn - junction diode shown in figure below?
A)
Zero
done
clear
B)
10 mA
done
clear
C)
20 mA
done
clear
D)
50 mA
done
clear
View Answer play_arrow
question_answer 15) The output form of a full wave rectifier is
A)
an AC voltage
done
clear
B)
a DC voltage
done
clear
C)
zero
done
clear
D)
a pulsating unidirectional voltage
done
clear
View Answer play_arrow
question_answer 16) Suitable impurities are added to a semiconductor depending on its use. This is done to
A)
increase its life
done
clear
B)
enable it to withstand high voltage
done
clear
C)
increase its electrical conductivity
done
clear
D)
increase its electrical resistivity
done
clear
View Answer play_arrow
question_answer 17) Absorption of X-rays is maximum in which of the following material sheets of same thickness?
A)
Cu
done
clear
B)
Au
done
clear
C)
Be
done
clear
D)
Pb
done
clear
View Answer play_arrow
question_answer 18) Lenz's law is a consequence of the law of conservation of
A)
charge
done
clear
B)
mass
done
clear
C)
momentum
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 19) A magnetic needle is kept in a non-uniform magnetic field. It experience
A)
a force only but not a torque
done
clear
B)
a force and torque both
done
clear
C)
a torque only but not a force
done
clear
D)
neither a torque nor a force
done
clear
View Answer play_arrow
question_answer 20) The magnitude of magnetic induction for a current carrying toroid of uniform cross - section is
A)
uniform over the whole cross-section
done
clear
B)
maximum on the outer edge
done
clear
C)
maximum on the inner edge
done
clear
D)
maximum at the centre of cross-section
done
clear
View Answer play_arrow
question_answer 21) Isogonic lines are those for which
A)
declination is the same at all places on the line
done
clear
B)
angle of dip is the same at the place on die line
done
clear
C)
die value of horizontal component of earth's magnetic field is the same
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 22) An electric current passes through a long straight wire. At a distance 5 cm from the wire, the magnetic field is B. The field at 20 cm from the wire would be
A)
2 B
done
clear
B)
B/4
done
clear
C)
B/2
done
clear
D)
B
done
clear
View Answer play_arrow
question_answer 23) An ammeter and a voltmeter of resistance R are connected in series to an electric cell of negligible internal resistance. Their readings are A and V respectively. If another resistances is connected in parallel with the voltmeter, then
A)
both A and V will increase
done
clear
B)
both A and V will decrease
done
clear
C)
A will decrease and V will increase
done
clear
D)
A will increase and V will decrease
done
clear
View Answer play_arrow
question_answer 24) The core of transformer is laminated to reduce the effect of
A)
copper losses
done
clear
B)
flux leakage
done
clear
C)
hysteresis loss
done
clear
D)
eddy current
done
clear
View Answer play_arrow
question_answer 25) The average power dissipation in pure inductance is
A)
\[\frac{1}{2}L{{I}^{2}}\]
done
clear
B)
\[2L{{I}^{2}}\]
done
clear
C)
\[\frac{1}{4}L{{I}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 26) The charge given to any conductor resides on its outer surface, because
A)
the free charge tends to be in its minimum potential energy state
done
clear
B)
the free charge tends to be in its minimum kinetic energy state
done
clear
C)
the free charge tends to be in its maximum potential energy state
done
clear
D)
the free charge tends to be in its maximum kinetic energy state
done
clear
View Answer play_arrow
question_answer 27) identical mercury droplets charged to the same potential V coalesce to form a single bigger drop. The potential of new drop will be
A)
\[\frac{V}{n}\]
done
clear
B)
nV
done
clear
C)
nV2
done
clear
D)
n2/3 V
done
clear
View Answer play_arrow
question_answer 28) For protecting sensitive equipment from external magnetic field, it should be
A)
wrapped with insulation around it when passing current through it
done
clear
B)
placed inside an iron can
done
clear
C)
surrounded with Cu sheet
done
clear
D)
placed inside aluminium can
done
clear
View Answer play_arrow
question_answer 29) The potential difference across the terminals of a battery is 50 V when 11 A current is drawn and 60 V when 1 A current is drawn. The emf and the internal resistance of the battery are
A)
62 V, 2\[\Omega \]
done
clear
B)
63 V, 1\[\Omega \]
done
clear
C)
61 V, 1\[\Omega \]
done
clear
D)
64 V, 2\[\Omega \]
done
clear
View Answer play_arrow
question_answer 30) Four resistances 100, 50, 70 and 30 are connected so that they form the sides of a rectangle AB, BC, CD, and DA respectively. Another resistance of 100 is connected across the diagonal AC. The equivalent resistance between A and B is
A)
20
done
clear
B)
50
done
clear
C)
7 ft
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 31) The potential energy of a charged parallel plate capacitor is U0. If a slab of dielectric constant K is inserted between the plates, then the new potential energy will be
A)
\[\frac{{{U}_{0}}}{K}\]
done
clear
B)
\[{{U}_{0}}{{K}^{2}}\]
done
clear
C)
\[\frac{{{U}_{0}}}{{{K}^{2}}}\]
done
clear
D)
\[{{U}_{0}}^{2}\]
done
clear
View Answer play_arrow
question_answer 32) Two similar heater coils separately take 10 min to boil a certain amount of water. If both coils are connected in series, time taken to boil the same amount of water will be
A)
15 min
done
clear
B)
20 min
done
clear
C)
7.5 min
done
clear
D)
25 min
done
clear
View Answer play_arrow
question_answer 33) Same current is being passed through a copper voltameter and a silver voltameter. The rate of increase in weights of the cathode of the two voltameters will be proportional to
A)
atomic masses
done
clear
B)
atomic number
done
clear
C)
relative densities
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 34) Two equal and opposite charge (+ q and - q) are situated at x distance from each other, the value of potential at very far point will depend upon
A)
only on q
done
clear
B)
only on x
done
clear
C)
on qx
done
clear
D)
on \[\frac{q}{x}\]
done
clear
View Answer play_arrow
question_answer 35) In a potentiometer of one- metre length, an unknown emf voltage source is balanced at 60 cm length of potentiometer wire, while a 3 V battery is balanced at 45 cm length. Then the emf of the unknown voltage source is
A)
3 V
done
clear
B)
2.25 V
done
clear
C)
4 V
done
clear
D)
4.5 V
done
clear
View Answer play_arrow
question_answer 36) A car travelling on a straight path moves with uniform velocity v1 for some time and with velocity v2 for next equal time, the average velocity is given by
A)
\[\sqrt{{{v}_{1}}{{v}_{2}}}\]
done
clear
B)
\[\left( \frac{{{v}_{1}}+{{v}_{2}}}{2} \right)\]
done
clear
C)
\[{{\left( \frac{1}{{{v}_{1}}}+\frac{1}{{{v}_{2}}} \right)}^{-1}}\]
done
clear
D)
\[2{{\left( \frac{1}{{{v}_{1}}}+\frac{1}{{{v}_{2}}} \right)}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 37) A particle of mass m moves in a circular path radius r under the action of a force \[\frac{m{{v}^{2}}}{r}\].The work done during its motion over half of the circumference of the circular path will be
A)
\[\left( \frac{m{{v}^{2}}}{r} \right)\times 2\pi r\]
done
clear
B)
\[\left( \frac{m{{v}^{2}}}{r} \right)\times \pi r\]
done
clear
C)
\[\frac{\left( 2\pi r \right)}{\left( \frac{m{{v}^{2}}}{r} \right)}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) Dimensions of self inductance are
A)
[MLT-2A-3]
done
clear
B)
[ML-2T-1A-2]
done
clear
C)
[ML2 T-2 A-2]
done
clear
D)
[ML2T-2A-1]
done
clear
View Answer play_arrow
question_answer 39) A car of mass m is moving with momentum p. If\[\mu \] be the coefficient of friction between the tyres and the road, what will be stopping distance due to friction alone?
A)
\[\frac{{{p}^{2}}}{2\mu g}\]
done
clear
B)
\[\frac{{{p}^{2}}}{2m\mu g}\]
done
clear
C)
\[\frac{{{p}^{2}}}{2{{m}^{2}}\mu g}\]
done
clear
D)
\[\frac{{{p}^{2}}}{2mg}\]
done
clear
View Answer play_arrow
question_answer 40) A neutron is moving with velocity u. It collides head on and elastically with an atom of mass number A. If the initial kinetic energy of the neutron be E, how much kinetic energy will be retained by the neutron after collision?
A)
\[{{\left( \frac{A}{A+1} \right)}^{2}}E\]
done
clear
B)
\[\frac{A}{{{\left( A+1 \right)}^{2}}}E\]
done
clear
C)
\[{{\left( \frac{A-1}{A+1} \right)}^{2}}E\]
done
clear
D)
\[\frac{A-1}{{{\left( A+1 \right)}^{2}}}E\]
done
clear
View Answer play_arrow
question_answer 41) If the momentum of a particle is increased by 20%, then its kinetic energy increases by
A)
44%
done
clear
B)
66%
done
clear
C)
80%
done
clear
D)
30%
done
clear
View Answer play_arrow
question_answer 42) Three point masses, each of mass M are placed at the comers of an equilateral triangle of side L. The moment of inertia of this system about an axis along one side of the triangle is
A)
\[\frac{1}{3}M{{L}^{2}}\]
done
clear
B)
\[\frac{3}{2}M{{L}^{2}}\]
done
clear
C)
\[\frac{3}{4}M{{L}^{2}}\]
done
clear
D)
ML2
done
clear
View Answer play_arrow
question_answer 43) A thin circular ring of mass M and radius R is rotating about its axis with a constant angular velocity\[\omega \]. Two objects, each of mass m, are connected gently to the ring. The ring now rotates with an angular velocity
A)
\[\frac{\omega M}{M+m}\]
done
clear
B)
\[\frac{\omega \left( M-2M \right)}{\left( M+2m \right)}\]
done
clear
C)
\[\frac{\omega \left( M+2m \right)}{M}\]
done
clear
D)
\[\frac{\omega M}{M+2m}\]
done
clear
View Answer play_arrow
question_answer 44) A satellite of mass m is moving in a circular orbit of radius R above the surface of a planet of mass M and radius R. The amount of work done to shift the satellite to higher orbit of radius is
A)
\[mgR\]
done
clear
B)
\[\frac{mgR}{6}\]
done
clear
C)
\[\frac{mMgR}{\left( M+m \right)}\]
done
clear
D)
\[\frac{mMgR}{6\left( M+m \right)}\]
done
clear
View Answer play_arrow
question_answer 45) In a gravitational force field a particle is taken from A to B along different paths as shown in figure. Then
A)
work done along path I will be maximum
done
clear
B)
work done along path III wilt be maximum
done
clear
C)
work done along path IV will be maximum
done
clear
D)
work done along all the paths will be the same
done
clear
View Answer play_arrow
question_answer 46) A wire of length L and area of cross-section A is made of material of Young's modulus Y. If the wire is stretched by the amount x, the work done is
A)
\[\frac{YA{{x}^{2}}}{2L}\]
done
clear
B)
\[YA{{x}^{2}}L\]
done
clear
C)
\[\frac{YAx}{2L}\]
done
clear
D)
\[\frac{YA{{x}^{2}}}{L}\]
done
clear
View Answer play_arrow
question_answer 47) The potential energy of a molecule increases when it is brought to the surface from the interior of a liquid because
A)
at the free liquid surface gravitational potential energy is more
done
clear
B)
work has to be done to move a molecule to the surface against the repulsive component of the inter molecular forces
done
clear
C)
work has to be done to move a molecule to the surface against the attraction from Other molecules
done
clear
D)
the temperature of the liquid surface is always more than that of the interior of the liquid
done
clear
View Answer play_arrow
question_answer 48) When a van der Waals' gas undergoes free expansion then its temperature
A)
decreases
done
clear
B)
increases
done
clear
C)
does not change
done
clear
D)
depends upon the nature of the gas
done
clear
View Answer play_arrow
question_answer 49) A cylinder of radius r and of thermal conductivity K1 is surrounded by a cylindrical shell of inner radius r and outer radius 2r made of a material of thermal conductivity K2. The effective thermal conductivity of the system if
A)
\[\frac{1}{3}\left( {{K}_{1}}+2{{K}_{2}} \right)\]
done
clear
B)
\[\frac{1}{2}\left( 2{{K}_{1}}+3{{K}_{2}} \right)\]
done
clear
C)
\[\frac{1}{4}\left( 3{{K}_{2}}+2{{K}_{1}} \right)\]
done
clear
D)
\[\frac{1}{4}\left( {{K}_{1}}+3{{K}_{2}} \right)\]
done
clear
View Answer play_arrow
question_answer 50) The tungsten filament of an electric lamp has a surface area A and a power rating P. If the emissivity of the filament is i; and a is Stefan's constant, the steady temperature of the filament will be
A)
\[T={{\left( \frac{P}{A\,\varepsilon \,\sigma } \right)}^{4}}\]
done
clear
B)
\[T=\left( \frac{P}{A\,\varepsilon \,\sigma } \right)\]
done
clear
C)
\[T={{\left( \frac{A\,\varepsilon \,\sigma }{P} \right)}^{\frac{1}{4}}}\]
done
clear
D)
\[T={{\left( \frac{P}{A\,\varepsilon \,\sigma } \right)}^{\frac{1}{4}}}\]
done
clear
View Answer play_arrow
question_answer 51) The pH value of \[1\times {{10}^{-4}}\text{M}\,\text{NAOH}\]solution is
A)
4
done
clear
B)
10
done
clear
C)
6
done
clear
D)
between 6-7
done
clear
View Answer play_arrow
question_answer 52) The C?H bond distance is the longest in
A)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}B{{r}_{2}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
View Answer play_arrow
question_answer 53) Which represents the correct order of first ionisation potential of third period elements?
A)
\[Na\text{ }>\text{ }Mg\text{ }>\text{ }Al\text{ }>\text{ }Si\]
done
clear
B)
\[Na\text{ }<\text{ }Mg\text{ }<\text{ }Al\text{ }<\text{ }Si\]
done
clear
C)
\[Na\text{ }<\text{ }Si\text{ }<\text{ }Al\text{ }<\text{ }Mg\]
done
clear
D)
\[Na\text{ }<\text{ }Al\text{ }<\text{ }Mg\text{ }<\text{ }Si\]
done
clear
View Answer play_arrow
question_answer 54) By which of the following processes, pure nitrogen gas is prepared?
A)
\[(N{{H}_{4}})C{{r}_{2}}{{O}_{7}}\xrightarrow{\Delta }\]
done
clear
B)
\[N{{H}_{4}}Cl+NaN{{O}_{2}}\xrightarrow{\Delta }\]
done
clear
C)
\[N{{H}_{3}}+NaN{{O}_{2}}\xrightarrow{\Delta }\]
done
clear
D)
\[{{N}_{2}}O+Cu\xrightarrow{\Delta }\]
done
clear
View Answer play_arrow
question_answer 55) In the following reaction, \[{{C}_{2}}{{H}_{5}}OH+C{{H}_{3}}COOH\xrightarrow[-{{H}_{2}}O]{Conc.{{H}_{2}}S{{O}_{4}}}\] \[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\] \[{{C}_{2}}{{H}_{5}}OH\]acts as
A)
electrophile
done
clear
B)
nucleophile
done
clear
C)
dehydrating agent
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 56) Which of the following orbital diagram violates Faults exclusion principle?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 57) The geometry of sulphate ion is
A)
square planar
done
clear
B)
tetrahedral
done
clear
C)
square pyramidal
done
clear
D)
octahedral
done
clear
View Answer play_arrow
question_answer 58) The difference between heat capacity at constant pressure and heat capacity at constant volume for the combustion of carbon monoxide at \[27{{\,}^{o}}C\] will be
A)
\[~-\text{ }124.71\text{ kJ}\]
done
clear
B)
\[-\text{ }1.247\text{ J}\]
done
clear
C)
\[~-\text{ }1.247\text{ kJ}\]
done
clear
D)
\[-\text{ }124.71\text{ J}\]
done
clear
View Answer play_arrow
question_answer 59) IUPAC name of the given compound is \[{{H}_{3}}C-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,=\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,-COOH\]
A)
2-methylbut-2-enoic acid
done
clear
B)
3-methylbut-2-enoic acid
done
clear
C)
3-methylbut-3-enoic acid
done
clear
D)
2-methylbut-3-enoic acid
done
clear
View Answer play_arrow
question_answer 60) Which of the following is an aromatic compound?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 61) Thermodynamically, most stable form of phosphorus is
A)
red
done
clear
B)
black
done
clear
C)
white
done
clear
D)
yellow
done
clear
View Answer play_arrow
question_answer 62) The substance with the highest calorific value is
A)
milk
done
clear
B)
rice
done
clear
C)
ghee
done
clear
D)
egg
done
clear
View Answer play_arrow
question_answer 63) Gamma rays are
A)
high energy electrons
done
clear
B)
low energy electrons
done
clear
C)
high energy electro-magnetic waves
done
clear
D)
high energy positrons
done
clear
View Answer play_arrow
question_answer 64) What volume of \[C{{O}_{2}}\] will be liberated at NTP if 12 g of carbon is burnt in excess of oxygen?
A)
11.2L
done
clear
B)
22.4 L
done
clear
C)
2.24 L
done
clear
D)
1.12 L
done
clear
View Answer play_arrow
question_answer 65) Which of the following is man-made element?
A)
Ra
done
clear
B)
U
done
clear
C)
Np
done
clear
D)
C
done
clear
View Answer play_arrow
question_answer 66) Four different colloids have the following Gold number. Which one has its most effective action?
A)
10
done
clear
B)
30
done
clear
C)
20
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 67) The number of unpaired electrons in ferrous ion is
A)
3
done
clear
B)
2
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 68) Ascorbic acid is the chemical name of
A)
vitamin\[{{B}_{6}}\]
done
clear
B)
vitamin A
done
clear
C)
vitamin C
done
clear
D)
vitamin D
done
clear
View Answer play_arrow
question_answer 69) Which amine of the following will not answer carbylamine reaction?
A)
Ethyl amine
done
clear
B)
Methyl amine
done
clear
C)
Dimethyl amine
done
clear
D)
Phenyl amine
done
clear
View Answer play_arrow
question_answer 70) For a reaction, the dimensions of rate constant are same as that of rate, hence order of reaction is
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 71) The concentration (in mol/L) of the solution having osmotic pressure 0.0821 atm at 300 K will be
A)
0.33
done
clear
B)
0.066
done
clear
C)
\[0.3\times {{10}^{-2}}\]
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 72) Given, \[P{{b}^{2+}}/Pb=-0.126V;\]\[Z{{n}^{2+}}/Zn=-0.763\,\,V\] Find the emf of the following cell \[Zn|Z{{n}^{2+}}(0.1\,M)||P{{b}^{2+}}(1M)Pb.\]
A)
\[-\text{ }0.637\]
done
clear
B)
+ 0.637
done
clear
C)
> 0.637
done
clear
D)
+ 0.889
done
clear
View Answer play_arrow
question_answer 73) Which is the most abundant metal in the earthy crust?
A)
Fe
done
clear
B)
Al
done
clear
C)
Ca
done
clear
D)
Na
done
clear
View Answer play_arrow
question_answer 74) A hydrocarbon has carbon and hydrogen. Its molecular weight is 28. Its possible formula would be
A)
\[{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[{{C}_{4}}{{H}_{8}}\]
done
clear
View Answer play_arrow
question_answer 75) Which is an example of thermosetting polymer?
A)
Polythene
done
clear
B)
PVC
done
clear
C)
Neoprene
done
clear
D)
Bakelite
done
clear
View Answer play_arrow
question_answer 76) The first Noble Prize in chemistry was given to
A)
J.H. van' t Hoff
done
clear
B)
Cannizaro
done
clear
C)
Mendeleef
done
clear
D)
Moseley
done
clear
View Answer play_arrow
question_answer 77) Strongest reducing agent is
A)
K
done
clear
B)
Mg
done
clear
C)
Al
done
clear
D)
Ba
done
clear
View Answer play_arrow
question_answer 78) The base found only in the nucleotldes of RNA, is
A)
adenine
done
clear
B)
uracil
done
clear
C)
guanine
done
clear
D)
cytosine
done
clear
View Answer play_arrow
question_answer 79) Which of the following compounds does not give a precipitate with excess of NaOH?
A)
\[ZnS{{O}_{4}}\]
done
clear
B)
\[FeS{{O}_{4}}\]
done
clear
C)
\[AgN{{O}_{3}}\]
done
clear
D)
\[HgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) Among the following, the correct statement is
A)
aniline is a weaker base than ammonia
done
clear
B)
in water, solubility of\[C{{H}_{3}}OH>{{C}_{2}}{{H}_{5}}OH>{{C}_{6}}{{H}_{5}}OH\]
done
clear
C)
b. p. of alkylhalide is greater than its corresponding alkane
done
clear
D)
All of the given statements are correct
done
clear
View Answer play_arrow
question_answer 81) Three products are obtained by the ozonolysis of penta-1, 3-diene. Out of these if two products are formaldehyde and acetaldehyde, the name of the third one is
A)
formaldehyde
done
clear
B)
ethanol
done
clear
C)
glyoxal
done
clear
D)
propanaldehyde
done
clear
View Answer play_arrow
question_answer 82) \[C{{H}_{2}}=CH-C{{H}_{2}}-CH=C{{H}_{2}}\] represents a/an
A)
conjugated system
done
clear
B)
cumulative system
done
clear
C)
isolated system
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 83) In the presence of a catalyst, activation energy of a reaction is lowered by 2 kcal at \[27{{\,}^{o}}C.\] Hence, rate will be
A)
20 times
done
clear
B)
28 times
done
clear
C)
14 times
done
clear
D)
remain the same
done
clear
View Answer play_arrow
question_answer 84) The number of metamers of the compound with molecular formula \[{{C}_{5}}{{H}_{10}}O\]is
A)
1
done
clear
B)
3
done
clear
C)
8
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 85) The compound, with which ethanal does not react, is
A)
\[HCl\]
done
clear
B)
\[C{{l}_{2}}\]
done
clear
C)
\[PC{{l}_{5}}\]
done
clear
D)
\[aq\text{ }NaHS{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 86) The incorrect order with respect to the acidic strength is
A)
formic acid > acetic acid > propionic acid
done
clear
B)
cyclohexanol < phenol < benzoic acid
done
clear
C)
benzamide < aniline < cyclohexylamine
done
clear
D)
\[FC{{H}_{2}}COOH>ClC{{H}_{2}}COOH>BrC{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 87)
A)
acetic acid
done
clear
B)
formaldehyde
done
clear
C)
formic acid
done
clear
D)
propionic acid
done
clear
View Answer play_arrow
question_answer 88) \[\text{HCl}\]molecule contains
A)
ionic bond
done
clear
B)
covalent bond
done
clear
C)
hydrogen bond
done
clear
D)
coordinate bond
done
clear
View Answer play_arrow
question_answer 89) Transition metals show paramagnetic behaviour. This is because of their
A)
high lattice energy
done
clear
B)
variable oxidation state
done
clear
C)
characteristic configuration
done
clear
D)
unpaired electrons
done
clear
View Answer play_arrow
question_answer 90) Which reaction is not affected by change in pressure?
A)
\[{{H}_{2}}+{{I}_{2}}\rightleftharpoons 2HI\]
done
clear
B)
\[2C+{{O}_{2}}\rightleftharpoons 2CO\]
done
clear
C)
\[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}\]
done
clear
D)
\[PC{{l}_{5}}\rightleftharpoons PC{{l}_{3}}+C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 91) If the solubility of calcium fluoride in pure water is \[x\]mol/L, its solubility product is
A)
\[\sqrt{2}x\]
done
clear
B)
\[2{{x}^{2}}\]
done
clear
C)
\[4{{x}^{3}}\]
done
clear
D)
\[{{x}^{2}}\]
done
clear
View Answer play_arrow
question_answer 92) The molarity of a solution containing 5.0 g of \[\text{NaOH}\] in 250 mL solution is
A)
0.1
done
clear
B)
0.5
done
clear
C)
1.0
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 93)
The following reaction is called Friedel-Craft's reaction.
A)
\[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-Cl\]
done
clear
B)
\[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-R\]
done
clear
C)
\[R-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-H\]
done
clear
D)
\[R-O-R\]
done
clear
View Answer play_arrow
question_answer 94) Picric acid is
A)
2, 4, 6-tribromophenol
done
clear
B)
2, 4, 6-trinitrotoluene
done
clear
C)
2, 4, 6-trinitrophenol
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 95) Gravity separation process is used for the concentration of
A)
calamine
done
clear
B)
haematite
done
clear
C)
chalcopyrite
done
clear
D)
bauxite
done
clear
View Answer play_arrow
question_answer 96) Ammonium ion is
A)
a conjugate acid.
done
clear
B)
a conjugate base
done
clear
C)
neither an acid nor a base
done
clear
D)
both an acid and a base
done
clear
View Answer play_arrow
question_answer 97) The equivalent weight of \[\text{MnS}{{\text{O}}_{\text{4}}}\]is half of its molecular weight when it is converted to
A)
\[\text{M}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]
done
clear
B)
\[\text{Mn}{{\text{O}}_{\text{2}}}\]
done
clear
C)
\[\text{MnO}_{4}^{-}\]
done
clear
D)
\[MnO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 98) The reaction by which benzaldehyde is converted in benzyl alcohol, is
A)
Fittig reaction
done
clear
B)
Cannizaro reaction
done
clear
C)
Wurtz reaction
done
clear
D)
aldol condensation
done
clear
View Answer play_arrow
question_answer 99) By the ideal gas law, the pressure of 0.60 mole \[\text{N}{{\text{H}}_{\text{3}}}\]gas in a 3.00 L vessel at \[25{{\,}^{o}}C\] is
A)
48.9 atm
done
clear
B)
4.89 atm
done
clear
C)
0.489 arm
done
clear
D)
489 atm
done
clear
View Answer play_arrow
question_answer 100) Brown ring in the test of nitrate ion is btained due to the formation of
A)
\[[Fe{{({{H}_{2}}O)}_{5}}NO]S{{O}_{4}}\]
done
clear
B)
\[[Fe{{(S{{O}_{4}})}_{2}}NO]{{H}_{2}}O\]
done
clear
C)
\[F{{e}_{2}}{{(S{{O}_{4}})}_{3}}.NO\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 101) Energy flow in an ecosystem is
A)
unidirectional
done
clear
B)
bidirectional
done
clear
C)
multi-directional
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 102) Who proposed a five-kingdom classification and named kingdoms as Monera, Protista, Fungi, Plantae and Animalia?
A)
Herbert Copeland
done
clear
B)
R H Whittaker
done
clear
C)
Carl Woese
done
clear
D)
Carolus Linnaeus
done
clear
View Answer play_arrow
question_answer 103) Which of the following organisms completely lack cell wall, they are the smallest living cells known and can survive without oxygen?
A)
Mycoplasma
done
clear
B)
Euglenoids
done
clear
C)
Slime moulds
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 104) What is the correct order of the stages of cellular respiration?
A)
Krebs cycleelectron - transport - chain glycolysis
done
clear
B)
Electron - Krebs cycle - glycolysis transport chain
done
clear
C)
Glycolysis - Krebs cycle - electron transportchain
done
clear
D)
Glycolysis - electron - Krebs cycle transportchain
done
clear
View Answer play_arrow
question_answer 105) Who discovered an infectious agent that was to be a free RNA of low molecular weight, it also lacked protein coat. This agent caused potato spindle tuber disease?
A)
M W Beijerinck
done
clear
B)
T O Diener
done
clear
C)
J Ivanowsky
done
clear
D)
W M Stanley
done
clear
View Answer play_arrow
question_answer 106) Bioluminescence is well marked in
A)
flatworms
done
clear
B)
ctenophores
done
clear
C)
cnidaria
done
clear
D)
aschelminthes
done
clear
View Answer play_arrow
question_answer 107) Small disc-shaped structures at the surface of the centromeres that appear during metaphase are
A)
kinetochores
done
clear
B)
metaphase plate
done
clear
C)
spindlefibres
done
clear
D)
chromatid
done
clear
View Answer play_arrow
question_answer 108) In some organisms, karyokinesis is not followed by cytokinesis as a result of which, multinucleate condition arises leading to the formation of syncytium. The perfect example for this is
A)
appearance of a furrow in cell membrane
done
clear
B)
liquid endosperm in coconut
done
clear
C)
sexual reproduction
done
clear
D)
fertilization
done
clear
View Answer play_arrow
question_answer 109) When a molecule moves across a membrane independent of other molecules, the process is called
A)
uniport
done
clear
B)
symport
done
clear
C)
antiport
done
clear
D)
facilitated diffusion
done
clear
View Answer play_arrow
question_answer 110) Golden rice was created by transforming rice with two beta-carotene biosynthesis genes, namely,
A)
\[\text{Psy}\]and \[\text{Crt 1}\]genes
done
clear
B)
\[\text{LCY}-\text{e}\]
done
clear
C)
\[\text{CHY}-\text{1}\]
done
clear
D)
\[\text{CHY}-2\]
done
clear
View Answer play_arrow
question_answer 111) A scientist added a chemical (cyanide) to an animal cell to stop aerobic respiration. Which of the following is most likely to have been affected by this treatment?
A)
Active transport of substances across the plasma membrane
done
clear
B)
Passive transport of substances across the plasma membrane
done
clear
C)
Diffusion of substances across the plasma membrane
done
clear
D)
The thickness of the plasma membrane
done
clear
View Answer play_arrow
question_answer 112) Mobilization of stored food in germinating seed is triggered by
A)
ABA
done
clear
B)
GA
done
clear
C)
cytokinin
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 113) In the cell mediated immuno response, T-lymphocytes divide and secrete
A)
antigens
done
clear
B)
plasmogens
done
clear
C)
collagens
done
clear
D)
cytokines
done
clear
View Answer play_arrow
question_answer 114) Which of the following elements is an activator for both ribulosebiphosphate carboxylase oxygenase and phosphoenol pyruvate carboxylase in photosynthetic carbon fixation?
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[Z{{n}^{2+}}\]
done
clear
C)
\[C{{a}^{2+}}\]
done
clear
D)
\[S{{O}_{4}}^{2-}\]
done
clear
View Answer play_arrow
question_answer 115) The inward movement of ions into the cells is ......and the outward movement is......
A)
influx; efflux
done
clear
B)
efflux; influx
done
clear
C)
absorption; adsorption
done
clear
D)
adsorption; absorption
done
clear
View Answer play_arrow
question_answer 116) Which of the PGR6 induces pathenocarpy in tomatoes?
A)
Auxin
done
clear
B)
Gibberellin
done
clear
C)
Cytokinin
done
clear
D)
Ethylene
done
clear
View Answer play_arrow
question_answer 117) Complete the equation : \[\text{Nucleic acids}\xrightarrow[{}]{\text{Nucleases}}\text{Nucleotides }\xrightarrow[{}]{{}}......\]
A)
Monoglycerides
done
clear
B)
Diglycerides
done
clear
C)
Disaccharides
done
clear
D)
Nucleosides
done
clear
View Answer play_arrow
question_answer 118) Which of the following is an organic molecule needed by the body in small amounts?
A)
Protein
done
clear
B)
Zinc
done
clear
C)
Vitamin-C
done
clear
D)
Monosaccharide
done
clear
View Answer play_arrow
question_answer 119) Which of the following rule states that animals of colder areas have shorter tails as compared to animals of warmer areas?
A)
Allen's rule
done
clear
B)
Rensch's rule
done
clear
C)
Jordan's rule
done
clear
D)
Bergman's rule
done
clear
View Answer play_arrow
question_answer 120) Secretin and cholecystokinin are digestive hormones. They are secreted in
A)
oesophagus
done
clear
B)
ileum
done
clear
C)
duodenum
done
clear
D)
pyloric
done
clear
View Answer play_arrow
question_answer 121) G-6-P dehydrogenase deficiency is associated with haemolysis of
A)
lymphocytes
done
clear
B)
RBCs
done
clear
C)
platelets
done
clear
D)
leucocytes
done
clear
View Answer play_arrow
question_answer 122) Biodiversity Act of India was passed by the Parliament in the year
A)
1996
done
clear
B)
1992
done
clear
C)
2002
done
clear
D)
2000
done
clear
View Answer play_arrow
question_answer 123) Chlorophyll in chloroplasts is located in
A)
grana
done
clear
B)
pyrenoid
done
clear
C)
stroma
done
clear
D)
Both (a) and (c)
done
clear
View Answer play_arrow
question_answer 124) The post Bhopal gas disaster analysis showed that the accident started, when the leakage of a tank started containing
A)
methylisocyanide
done
clear
B)
methylisocyanate
done
clear
C)
ethylisocyanide
done
clear
D)
ethylisocyanate
done
clear
View Answer play_arrow
question_answer 125) Which of the following herbicides and defoliant were used by the US military in its herbicidal warfare programme during the Vietnam war ?
A)
Agent black
done
clear
B)
Agent orange
done
clear
C)
Super orange
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 126) Some individuals with blood group A may inherit the genes for blond hair, while other individuals with blood group A may inherit the gene for brown hair. This can be best explained by the principle of
A)
dominance
done
clear
B)
multiple alleles
done
clear
C)
independent assortment
done
clear
D)
incomplete dominance
done
clear
View Answer play_arrow
question_answer 127) Which one of the following is correctly matched?
A)
National Institute of Virology?Pune
done
clear
B)
National institute of Communicable Diseases?Lucknow
done
clear
C)
Central Drug Research Institute?Kasauli
done
clear
D)
National Institute of Nutrition?Mumbai
done
clear
View Answer play_arrow
question_answer 128) Which of the following abnormalities results from an unnatural presence of a Barr body that it would normally not have?
A)
Turner's syndrome
done
clear
B)
Down's syndrome
done
clear
C)
Klinefelter's syndrome
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 129) If a single plant species is removed from a food web, then most likely
A)
an animal species will fill the unoccupied niche
done
clear
B)
other plants will produce enough food for herbivores
done
clear
C)
dependent herbivores will have to find new food sources
done
clear
D)
carnivores will be unaffected by the loss
done
clear
View Answer play_arrow
question_answer 130) A vaccine meant for protection against tuberculosis is
A)
BCG
done
clear
B)
DPT
done
clear
C)
TT
done
clear
D)
BGC
done
clear
View Answer play_arrow
question_answer 131) The patient of this disease exhibit memory loss (particularly for recent events), shortened attention span, disorientation and eventual language loss.
A)
Cancer
done
clear
B)
AIDS
done
clear
C)
Alzheimer's disease
done
clear
D)
Cystic fibrosis
done
clear
View Answer play_arrow
question_answer 132) This tumour normally confined to their original location and do not spread to other parts of the body.
A)
Malignant tumour
done
clear
B)
Metastasis
done
clear
C)
Benign tumour
done
clear
D)
Cancer
done
clear
View Answer play_arrow
question_answer 133) Certain bacteria living in the soil poor in oxygen, convert nitrates into nitrites and then to free nitrogen and such bacteria are termed as
A)
nitrogen fixing bacteria
done
clear
B)
denitrifying bacteria
done
clear
C)
ammonifying bacteria
done
clear
D)
saprophytic bacteria
done
clear
View Answer play_arrow
question_answer 134) Which of the following respiratory organs are present in spiders and scorpions?
A)
Book lungs
done
clear
B)
Gills
done
clear
C)
Gill books
done
clear
D)
Lungs
done
clear
View Answer play_arrow
question_answer 135) Who discovered that restriction enzymes have the capability of cutting DNA strands in a particular fashion, which left what has became known as ?sticky ends? on the strands ?
A)
Ramdeo Mishra
done
clear
B)
Stanley Cohen
done
clear
C)
Herbert Boyer
done
clear
D)
James D Watson
done
clear
View Answer play_arrow
question_answer 136) Formation of non functional methaemoglobin causes blue-baby syndrome. This is due to
A)
excess of arsenic concentration in drinking water
done
clear
B)
excess of nitrates in drinking water
done
clear
C)
deficiency of iron in food
done
clear
D)
increased methane content in the atmosphere
done
clear
View Answer play_arrow
question_answer 137) In maize, hybrid vigour is exploited by
A)
bombarding the seeds with DNA
done
clear
B)
crossing of two inbred parental lines
done
clear
C)
harvesting seeds from the most productive plants
done
clear
D)
inducing mutations
done
clear
View Answer play_arrow
question_answer 138) Hargobind Khorana also contributed to genetic engineering by synthesizing
A)
\[{{\text{p}}^{\text{BR322}}}\]
done
clear
B)
viroid
done
clear
C)
\[{{\text{p}}^{\text{BR42}}}\]
done
clear
D)
artificial gene
done
clear
View Answer play_arrow
question_answer 139) Which of the following is exotic species?
A)
Parthenium
done
clear
B)
Lantana
done
clear
C)
Eichhornia
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 140) The total number of biodiversity hot spots in the world are
A)
24
done
clear
B)
12
done
clear
C)
34
done
clear
D)
52
done
clear
View Answer play_arrow
question_answer 141) A bond formed between carboxyl group of one amino acid and amino group of adjacent amino acid is called
A)
peptide bond
done
clear
B)
hydrogen bond
done
clear
C)
covalent bond
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 142) When the body is rapidly oxidising fats, excess ketone bodies accumulate resulting in
A)
pyruvic acid
done
clear
B)
lactic acid
done
clear
C)
ketoacidosis
done
clear
D)
ATP
done
clear
View Answer play_arrow
question_answer 143) EERI is situated in
A)
Delhi
done
clear
B)
Mumbai
done
clear
C)
Nagpur
done
clear
D)
Bangaluru
done
clear
View Answer play_arrow
question_answer 144) A historic convention on biological diversity held in Rio de Janeiro in 1992 is known as
A)
The earth summit
done
clear
B)
Montreal protocol
done
clear
C)
Janeva convention
done
clear
D)
Janeiro convention
done
clear
View Answer play_arrow
question_answer 145) The flowers of Oxalis open during the day and close at night, such type of movement is
A)
photonasty
done
clear
B)
nyctinasty
done
clear
C)
photonastic
done
clear
D)
seismonastic
done
clear
View Answer play_arrow
question_answer 146) Silent valley is tropical evergreen forest located in
A)
Kerala
done
clear
B)
Karnataka
done
clear
C)
Maharashtra
done
clear
D)
Orissa
done
clear
View Answer play_arrow
question_answer 147) The results of Miller?s experiments were discussed in the book The Planets? written by
A)
Sayere
done
clear
B)
Harold Urey
done
clear
C)
Huxley
done
clear
D)
Stanley
done
clear
View Answer play_arrow
question_answer 148) Vitamin-C was the first vitamin to be produced by a fermentation process using
A)
Penicillium
done
clear
B)
E. coli
done
clear
C)
Yersinia pestis
done
clear
D)
Acetobacter
done
clear
View Answer play_arrow
question_answer 149) The functional unit of DNA molecule that codes for a particular gene product is
A)
cistron
done
clear
B)
exon
done
clear
C)
intron
done
clear
D)
gene
done
clear
View Answer play_arrow
question_answer 150) A gene that masks another gene?s expression is called
A)
dominant
done
clear
B)
recessive
done
clear
C)
epistatic
done
clear
D)
assorted
done
clear
View Answer play_arrow
question_answer 151) The largest public sector bank in India is
A)
State Bank of India
done
clear
B)
Allahabad Bank
done
clear
C)
Punjab National Bank
done
clear
D)
Indian Overseas bank
done
clear
View Answer play_arrow
question_answer 152) National Development Council was set up in
A)
1950
done
clear
B)
1951
done
clear
C)
1952
done
clear
D)
1953
done
clear
View Answer play_arrow
question_answer 153) One rupee currency note bears the signature of
A)
Prime Minister of India
done
clear
B)
President of India
done
clear
C)
Finance Minister of India
done
clear
D)
Finance Secretary of India
done
clear
View Answer play_arrow
question_answer 154) The first estimate of national income in India was made by
A)
Mahalonobis
done
clear
B)
V.K.R.V. Rao
done
clear
C)
Dada BhaiNaoroji
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 155) Who holds the power of Judicial review in India?
A)
The Parliament
done
clear
B)
The Minister of low
done
clear
C)
The Supreme Court and the High Court
done
clear
D)
Only Supreme Court
done
clear
View Answer play_arrow
question_answer 156) Who was the first Chairman of the Planning Commission?
A)
Gulzari Lai Nanda
done
clear
B)
J.L. Nehru
done
clear
C)
C.D. Deshmukh
done
clear
D)
K.C. Niyogy
done
clear
View Answer play_arrow
question_answer 157) Who presided as the temporary President of Constituent Assembly ?
A)
SacchidanandSinha
done
clear
B)
Dr. Rajendra Prasad
done
clear
C)
Dr. B.R. Ambedkar
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 158) What is the special constitutional position of Jammu & Kashmir?
A)
Indian laws are not applicable.
done
clear
B)
It has its own constitution.
done
clear
C)
It is not one of the integral parts of Indian union.
done
clear
D)
It is above the constitution
done
clear
View Answer play_arrow
question_answer 159) The 44th Amendment of the Indian Constitution withdrew the Fundamental Right
A)
to freedom of religion
done
clear
B)
to constitutional remedies
done
clear
C)
to property
done
clear
D)
against exploitation
done
clear
View Answer play_arrow
question_answer 160) In modern periodic table, 6th period contains
A)
32 elements
done
clear
B)
18 elements
done
clear
C)
30 elements
done
clear
D)
8 elements
done
clear
View Answer play_arrow
question_answer 161) What happens when steam is passed over red hot carbon?
A)
\[\text{C}{{\text{O}}_{\text{2}}}+{{\text{H}}_{\text{2}}}\] are formed
done
clear
B)
\[{{\text{H}}_{\text{2}}}+0\text{2}+\] steam are formed
done
clear
C)
\[\text{CO}+{{\text{H}}_{\text{2}}}\] are formed
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 162) Soda water contains
A)
nitrous acid
done
clear
B)
acetic acid
done
clear
C)
carbon dioxide
done
clear
D)
sulphuric acid
done
clear
View Answer play_arrow
question_answer 163) Lead pencil contains
A)
leadsulphate
done
clear
B)
lead
done
clear
C)
ferroussulphate
done
clear
D)
graphite
done
clear
View Answer play_arrow
question_answer 164) Which of the following has density greater than water?
A)
Li
done
clear
B)
Na
done
clear
C)
K
done
clear
D)
Cs
done
clear
View Answer play_arrow
question_answer 165) Glucagon is produced by
A)
peptic cells
done
clear
B)
oxyntic cells
done
clear
C)
alpha cells
done
clear
D)
beta cells
done
clear
View Answer play_arrow
question_answer 166) Inflorescence is
A)
group of flowers
done
clear
B)
occurrence of flowers
done
clear
C)
arrangement of flowers
done
clear
D)
arrangement of flowers on the floral axis
done
clear
View Answer play_arrow
question_answer 167) In which one of the following mammals do the ducts of the excretory system and genital tract have a common opening?
A)
Porcupine
done
clear
B)
Pangolin
done
clear
C)
Hedgehog
done
clear
D)
Echidna
done
clear
View Answer play_arrow
question_answer 168) Stem cutting are commonly used for the propagation of
A)
banana
done
clear
B)
rose
done
clear
C)
mango
done
clear
D)
cotton
done
clear
View Answer play_arrow
question_answer 169) Eating of raw fish can cause deficiency of vitamin
A)
D
done
clear
B)
\[{{\text{B}}_{\text{1}}}\]
done
clear
C)
\[{{\text{B}}_{4}}\]
done
clear
D)
\[{{\text{B}}_{12}}\]
done
clear
View Answer play_arrow
question_answer 170) Gandhiji was associated with the Peasant Movement of
A)
Bardoli
done
clear
B)
Champaran
done
clear
C)
Kheda
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 171) The Supreme Court in British India was established under
A)
The Charter Act of 1813
done
clear
B)
The Charter Act of 1833
done
clear
C)
Regulating Act of 1773
done
clear
D)
Pitt?s India Act of 1784
done
clear
View Answer play_arrow
question_answer 172) The total number of Purans are
A)
10
done
clear
B)
12
done
clear
C)
16
done
clear
D)
18
done
clear
View Answer play_arrow
question_answer 173) The biggest building at Mohenjodaro was the
A)
great granary
done
clear
B)
rectangular building
done
clear
C)
great bath
done
clear
D)
assembly hall
done
clear
View Answer play_arrow
question_answer 174) The earliest example of a land grant is provided by an inscription of
A)
the Mauryas
done
clear
B)
the Satvahans
done
clear
C)
the Guptas
done
clear
D)
the Chalukyas
done
clear
View Answer play_arrow
question_answer 175) Rabindranath Tagore returned the title of Knighthood to the British Government to protest against the
A)
Rowlatt Act of 1919
done
clear
B)
introduction of Diarchy in the provinces
done
clear
C)
partition of Bengal
done
clear
D)
massacre in JallianwallaBagh
done
clear
View Answer play_arrow
question_answer 176) Who was the first person to land on the moon?
A)
Neil Armstrong and Edwin Aldrin
done
clear
B)
Neelam Sanjeev Reddy and Einstein
done
clear
C)
Stephen Hawkins and Kingsley
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 177) The largest of Indian peninsular river is
A)
Ganga
done
clear
B)
Krishna
done
clear
C)
Godavari
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 178) The first person to conduct heart trans planation in India is
A)
Dr. P.K.K. Ayyangar
done
clear
B)
b) Dr. R. Valiathan
done
clear
C)
Dr. Vanugopal
done
clear
D)
Dr. R. Keshavan Nair
done
clear
View Answer play_arrow
question_answer 179) Which Indian became the President of International Court of Justice?
A)
Justice H.R. Khanna
done
clear
B)
Justice Ajit Ray
done
clear
C)
Dr. Nagendra Singh
done
clear
D)
Justice P.N. Bhagwati
done
clear
View Answer play_arrow
question_answer 180) ?Golan Heights? has become a controversial issue between which of the following pairs of nations?
A)
Iraq-Iran
done
clear
B)
Israel-Libya
done
clear
C)
Israel-Syria
done
clear
D)
Kuwait-Iraq
done
clear
View Answer play_arrow
question_answer 181) If? nitcoscotingo? means ?softer than flower? ; ?tingorhomst? means ?sweet flower fragrance? and ?mstscotmp? means ?sweet than smile?, what wuld ?fragrance? stand for ?
A)
rho
done
clear
B)
tmp
done
clear
C)
sco
done
clear
D)
mst
done
clear
View Answer play_arrow
question_answer 182) If \[x=\sqrt[3]{2\frac{93}{125}},\] then the value of x is
A)
\[2\frac{1}{5}\]
done
clear
B)
\[1\frac{2}{5}\]
done
clear
C)
\[3\frac{1}{5}\]
done
clear
D)
\[4\frac{1}{5}\]
done
clear
View Answer play_arrow
question_answer 183) The subduplicate ratio of \[16{{x}^{4}}:625{{y}^{6}}\] is
A)
\[\text{4}{{\text{x}}^{\text{2}}}:\text{25}{{\text{y}}^{\text{3}}}\]
done
clear
B)
\[~\text{4}{{\text{x}}^{\text{2}}}\text{:25}{{\text{y}}^{\text{2}}}\]
done
clear
C)
\[\text{4x}:\text{25y}\]
done
clear
D)
\[\text{8}{{\text{x}}^{\text{2}}}:\text{125}{{\text{y}}^{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 184) How many meters of cloth 50 m wide will be required to make a conical tent, the radius of whose base is 7 m and whose height is 24 m ?
A)
9 m
done
clear
B)
11m
done
clear
C)
12 m
done
clear
D)
13m
done
clear
View Answer play_arrow
question_answer 185) In a ratio which is equal to 7 : 8, if the antecedent is 35, the consequent is :
A)
30
done
clear
B)
32
done
clear
C)
36
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 186) Six persons are playing a game sitting in a circle facing the centre, Vijay was to the left of Sudhir. Amar was between Rakesh and Sarv. Neerav was second to the left of Amar. Which of the following is the position of Amar from Neerav?
A)
Second from right
done
clear
B)
Second to the left
done
clear
C)
Third from the left
done
clear
D)
Third from right
done
clear
View Answer play_arrow
question_answer 187) If ?nsoptrklichn? stands for ?Sapna gets marriage gift?, ?ptrInm wop chn? stands for ?wife gives marriage gift?, ?tti wop nhi? stands for ?he gives nothing?, what would means ?gives? ?
A)
wop
done
clear
B)
ptr
done
clear
C)
nhi
done
clear
D)
chn
done
clear
View Answer play_arrow
question_answer 188) Who is the tallest?
A)
Sunita
done
clear
B)
Bina
done
clear
C)
Gauri
done
clear
D)
Data inadequate
done
clear
View Answer play_arrow
question_answer 189) Who is the shortest?
A)
Radha
done
clear
B)
Renu
done
clear
C)
Seema
done
clear
D)
Data inadequate
done
clear
View Answer play_arrow
question_answer 190) What is the position of Radha from the shorter end?
A)
Fourth
done
clear
B)
Second
done
clear
C)
Third
done
clear
D)
Data inadequate
done
clear
View Answer play_arrow
question_answer 191) The most important use of fire for primitive man was
A)
to provide warmth
done
clear
B)
to provide light
done
clear
C)
to cook food
done
clear
D)
Both and
done
clear
View Answer play_arrow
question_answer 192) Primitive man used the fire-brand to
A)
keep away the wild animals
done
clear
B)
lessen the labour
done
clear
C)
provide light
done
clear
D)
prevent accidents
done
clear
View Answer play_arrow
question_answer 193) In the passage primary means
A)
primitive
done
clear
B)
elemental
done
clear
C)
fundamental
done
clear
D)
essential
done
clear
View Answer play_arrow
question_answer 194) Lamps, too, probably developed by accident. This statement shows that lamps developed through
A)
an accident
done
clear
B)
chance
done
clear
C)
planning
done
clear
D)
fate
done
clear
View Answer play_arrow
question_answer 195) Which of the following may be best title for the passage?
A)
Discovery of fire
done
clear
B)
Uses of fire
done
clear
C)
Primitive man and fire
done
clear
D)
Lamps
done
clear
View Answer play_arrow
question_answer 196) Select the appropriate world which is nearest in meaning to the word given in capitals. CONFIDENTIAL
A)
Secret
done
clear
B)
Private
done
clear
C)
Trusted
done
clear
D)
Hidden
done
clear
View Answer play_arrow
question_answer 197) Select the appropriate world which is nearest in meaning to the word given in capitals. MANY
A)
Endless
done
clear
B)
Several
done
clear
C)
Unlimited
done
clear
D)
Numberless
done
clear
View Answer play_arrow
question_answer 198) Select the appropriate world which is nearest in meaning to the word given in capitals. NIMBLE
A)
Lively
done
clear
B)
Clear
done
clear
C)
Quickening
done
clear
D)
Subtle
done
clear
View Answer play_arrow
question_answer 199) Select the appropriate world which is nearest in meaning to the word given in capitals. SANE
A)
Wild
done
clear
B)
Rational
done
clear
C)
Arrogant
done
clear
D)
Obscure
done
clear
View Answer play_arrow
question_answer 200) Select the appropriate world which is nearest in meaning to the word given in capitals. FATIGUE
A)
Sweating
done
clear
B)
Tension
done
clear
C)
Drowsiness
done
clear
D)
Weariness
done
clear
View Answer play_arrow
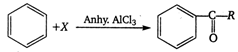 In this reaction ?X? is
In this reaction ?X? is
