A) 2
B) 3
C) 4
D) 5
Correct Answer: C
Solution :
| [c] Work done by gas |
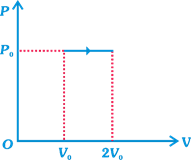
| \[={{P}_{0}}(2{{V}_{0}}-{{V}_{0}})={{P}_{0}}{{V}_{0}}\] ...(i) |
| Heat input to the gas |
| = Work done + Change in internal energy |
| \[={{P}_{0}}{{V}_{0}}+\frac{f}{2}nR\Delta T\] |
| \[={{P}_{0}}{{V}_{0}}+\frac{3}{2}(nR{{T}_{f}}-nR{{T}_{i}})\] |
| \[={{P}_{0}}{{V}_{0}}+\frac{3}{2}(2{{P}_{0}}{{V}_{0}}-{{P}_{0}}{{V}_{0}})\] |
| \[\therefore \] Heat input \[=\frac{5}{2}{{P}_{0}}{{V}_{0}}\] ....(ii) |
| Efficiency \[=\frac{\text{Work}\,\text{done}}{\text{Heat}\,\text{inpup}}\] |
| \[=\frac{{{P}_{0}}{{V}_{0}}}{\frac{5}{2}{{P}_{0}}{{V}_{0}}}=\frac{2}{5}\] |
| Efficiency \[=\frac{4}{10}=\frac{x}{10}\Rightarrow \,x=4\] |
You need to login to perform this action.
You will be redirected in
3 sec
