A) \[{{p}_{1}}<{{p}_{2}}\]
B) \[{{p}_{1}}>{{p}_{2}}\]
C) \[{{p}_{1}}={{p}_{2}}\]
D) Cannot predict
Correct Answer: A
Solution :
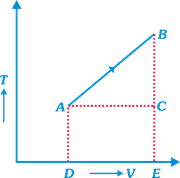
| [a] \[V=aT+b\] |
| From standard gas equation, |
| \[p=\frac{RT}{V}=\,\frac{RT}{aT+\,b}=\,\frac{R}{a+b/T}\] |
| As, \[{{T}_{2}}>{{T}_{1}}\] |
| \[{{p}_{2}}>{{p}_{1}}\] or \[{{p}_{1}}<{{p}_{2}}\]0 |
You need to login to perform this action.
You will be redirected in
3 sec
