| A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in figure. On the basis of this mark the correct option. |
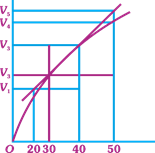 |
A) Average rate upto 40s is \[\frac{{{V}_{3}}-{{V}_{2}}}{40}\]
B) Average rate upto 40 seconds is \[\frac{{{V}_{3}}-{{V}_{2}}}{40-30}\]
C) Average rate upto 40 seconds is \[\frac{{{V}_{3}}}{40}\]
D) Average rate upto 40 seconds is \[\frac{{{V}_{3}}-{{V}_{1}}}{40-20}\]
Correct Answer: C
Solution :
Average rate \[=\frac{\Delta R}{\Delta t}=\frac{{{V}_{3}}}{40}\]You need to login to perform this action.
You will be redirected in
3 sec
