A) 0, 1, 2
B) 0, 0, 0
C) 1, 2, 3
D) 0, 2, 1
Correct Answer: B
Solution :
[b] \[PC{{l}_{5}}\Rightarrow \]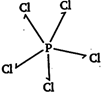 (no lone pair of electron)
(no lone pair of electron) 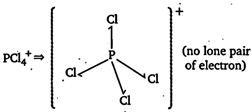
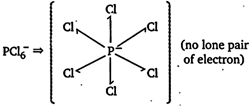
You need to login to perform this action.
You will be redirected in
3 sec
