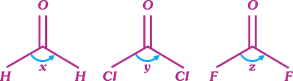
A) x > z > y
B) x > y > z
C) z > y > x
D) y > x > z
Correct Answer: B
Solution :
| [b] Idea |
| This problem includes conceptual mixing of bond angle of molecule and bent rule. |
| To solve this problem |
| -draw the structure of given compounds. |
| -apply bent rule and choose the correct answer. |
| Bent rule According to bent rule, |
| As the electronegativity of terminal atom increases, bond angle decrease. This is due to presence of more electron density towards electronegative atom. |
| Bond angle \[\frac{1}{\text{Electronegativity of terminal atom}}\] |
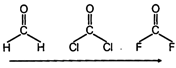 |
| Electronegativity of terminal atom increases. |
| Bond angle decreases. |
| TEST Edge Similar problems including bond angle, bond distance and reactivity of compound are also asked in JEE Main generally, So students are advised the study Bent's rule, VSEPR theory and drago rule also. |
You need to login to perform this action.
You will be redirected in
3 sec
