Answer:
(i) Extraction of Au (gold) by leaching with NaCN involves.
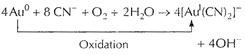
(a) Oxidation of gold (Au) to ![]() by
by ![]() (g)
(g)
![]() complexes
with cyanide
complexes
with cyanide ![]() forming
forming
![]() .
.
 (b) When complex is treated with active metal (as Zn),
pure metal is displaced. This involves reduction.
(b) When complex is treated with active metal (as Zn),
pure metal is displaced. This involves reduction.
![]() [1]
Thus, method involves redox reaction.
(ii) Powdered ore is suspended in water in a large vat,
together with suitable additives, (pine oil as frother and ethyl xanthate as
collector) and the mixture is agitated with air. Particles of ore become
attached to the air bubbles rise to the top of the vat and are collected in the
over flow froth. Particles of the undesired waste rock (gangue) fall to the
bottom. Thus, this is based on the difference in wet ability of different minerals.
[1]
[1]
Thus, method involves redox reaction.
(ii) Powdered ore is suspended in water in a large vat,
together with suitable additives, (pine oil as frother and ethyl xanthate as
collector) and the mixture is agitated with air. Particles of ore become
attached to the air bubbles rise to the top of the vat and are collected in the
over flow froth. Particles of the undesired waste rock (gangue) fall to the
bottom. Thus, this is based on the difference in wet ability of different minerals.
[1]
You need to login to perform this action.
You will be redirected in
3 sec
