Answer:
(a) (A)![]() (b)
(b)![]() (c)
(c)![]() (d)
(d)![]() (e)
(e)![]() [1]
Reactions
[1]
Reactions
![]() Orange in colour
Orange in colour
![]() (Calcium
nitride)
(Calcium
nitride)
![]() [1]
[1]
![]() (b)(i)
(b)(i)![]() (ii)
(ii)![]() Or
(a) (i) Solid
Or
(a) (i) Solid ![]() ionises
as
ionises
as ![]()
![]() Tetrahedral Octahedral [1]
(ii)
Tetrahedral Octahedral [1]
(ii) ![]() is
thermodynamic unstable since its decomposition into
is
thermodynamic unstable since its decomposition into ![]() is an
exothermic change.
is an
exothermic change.![]()
![]() Since, randomness increases due to increase in number of
molecules hence entropy also increases. We know
Since, randomness increases due to increase in number of
molecules hence entropy also increases. We know
![]()
![]() Thus,
Thus, ![]()
![]() Thus, conversion of
Thus, conversion of ![]() into
into ![]() is spontaneous
taking place with decrease in free energy. Hence,
is spontaneous
taking place with decrease in free energy. Hence, ![]() is
thermodynamic unstable. [1]
(iii) Most of the reactions of F^ are exothermic due to the
small and strong bond formation with other elements. [1]
is
thermodynamic unstable. [1]
(iii) Most of the reactions of F^ are exothermic due to the
small and strong bond formation with other elements. [1]
![]() (b) (i) Structure of
(b) (i) Structure of ![]()
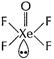 Square pyramidal,
Square pyramidal,![]() (ii) Structure of
(ii) Structure of ![]()
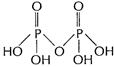 Each P-atom is sp3 hybridised.
Each P-atom is sp3 hybridised.
You need to login to perform this action.
You will be redirected in
3 sec
