Answer:
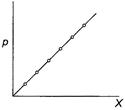
Henry's law states, "The partial pressure of the gas
in vapour phase (p) is proportional to the mole fraction of the gas (X)
in the solution."
![]() where,
where, ![]() is the
Henry's law constant.
If this is true variation of (p) with (X) can be
shown
is the
Henry's law constant.
If this is true variation of (p) with (X) can be
shown
 Applications
1. To increase the solubility of
Applications
1. To increase the solubility of ![]() in soft
drinks the bottle is sealed under high pressure.
2. In under water divers suffer from bends which are painful
and dangerous to life. To avoid these tanks used by divers are filled with air
diluted with He
in soft
drinks the bottle is sealed under high pressure.
2. In under water divers suffer from bends which are painful
and dangerous to life. To avoid these tanks used by divers are filled with air
diluted with He ![]() and
and
![]() [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
