Answer:
(a) (i)
Ethanol to acetone
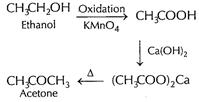 [1]
(ii)
Benzene to acetophenone
[1]
(ii)
Benzene to acetophenone
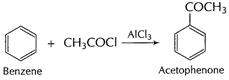 (iii)
Benzoic acid to benzaldehyde
(iii)
Benzoic acid to benzaldehyde
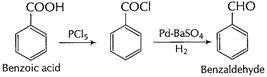 (b) (i)
Decarboxylation When sodium salt of a
carboxylic
acid is heated with sodalime, hydrocarbon is formed by loss of carbon
dioxide. It is called decarboxylation. Through it, carbon chain can be degraded
(b) (i)
Decarboxylation When sodium salt of a
carboxylic
acid is heated with sodalime, hydrocarbon is formed by loss of carbon
dioxide. It is called decarboxylation. Through it, carbon chain can be degraded
![]() [1]
[1]
![]() (ii)
Cannizzaro reaction Aldehydes without
(ii)
Cannizzaro reaction Aldehydes without ![]() react
with concentrated NaOH to give an acid salt by oxidation and an alcohol by
reduction. Thus disproportionate of aldehyde takes place. This is called
Cannizzaro reaction.
react
with concentrated NaOH to give an acid salt by oxidation and an alcohol by
reduction. Thus disproportionate of aldehyde takes place. This is called
Cannizzaro reaction.
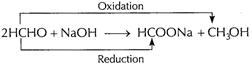 Benzaldehyde,
Benzaldehyde,
![]() without
without![]() also show
this reactions [1]
Or
(a)
also show
this reactions [1]
Or
(a)
[1]
Empirical
formula
%
Mol
Molar ratio
C
H
O
69.77
11.63
18.60
5.81
11.63
1.1625
5
10
0
![]() Empirical
formula weight = 60 +10 +16
=86
Molar mass
=86
Thus
molecular formula
Empirical
formula weight = 60 +10 +16
=86
Molar mass
=86
Thus
molecular formula ![]() It does not
reduce Tollen's reagent: CHO absent It forms bisulphite complex : keto group
It does not
reduce Tollen's reagent: CHO absent It forms bisulphite complex : keto group
![]() Thus, A
is
Thus, A
is![]() [1]
(b) (i)
[1]
(b) (i)
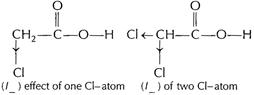 (/_) effect
of the two Cl-atoms stabilises the
(/_) effect
of the two Cl-atoms stabilises the ![]() to a
greater extent as compared to (/_) (/_) effect of one CI-atom. Thus
to a
greater extent as compared to (/_) (/_) effect of one CI-atom. Thus ![]() is weaker
than
is weaker
than ![]() .
[1]
(ii)
.
[1]
(ii)
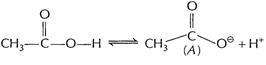 (A) is
stabilised by resonance in
(A) is
stabilised by resonance in ![]() only
only
![]() On the other
hand
On the other
hand![]() ionises
ionises
![]()
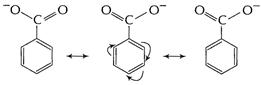
![]() is
stabilised in
is
stabilised in ![]() as
well as in
as
well as in ![]() .
.
You need to login to perform this action.
You will be redirected in
3 sec
