Answer:
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() Mole fraction in vapour phase
Mole fraction in vapour phase
![]()
![]() Or
1 molal solution in water
mole solute in 1000 g water
Or
1 molal solution in water
mole solute in 1000 g water
![]() (solute)
=1 mole
(solute)
=1 mole ![]() mole;
mole;
![]() (water)
(water)
![]()
![]()
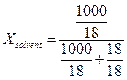 [1]
[1]
![]()
![]()
![]()
![]()
![]() [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
