Answer:
Salt is  , Plaster of Paris, white soft substance. (1)
It can be dough, moulded into different shapes, as 2 molecules of CaS04 share 1 molecule of
, Plaster of Paris, white soft substance. (1)
It can be dough, moulded into different shapes, as 2 molecules of CaS04 share 1 molecule of 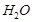 molecule. (1)
molecule. (1)
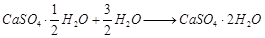 (1)
When it is left in open, it becomes solid mass
(1)
When it is left in open, it becomes solid mass 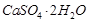 (gypsum) which cannot be used for moulding purposes as it is hard solid mass. (2)
Or
(a) The collection of all the processes involved in extracting metal from its ore is called metallurgy.
(b) (i) Froth-floatation process (ii) Roasting
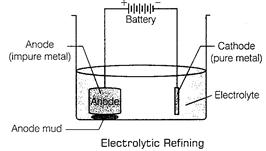
(c) Impure copper is taken as anode whereas pure copper is taken as cathode.
Copper sulphate solution
(gypsum) which cannot be used for moulding purposes as it is hard solid mass. (2)
Or
(a) The collection of all the processes involved in extracting metal from its ore is called metallurgy.
(b) (i) Froth-floatation process (ii) Roasting
(c) Impure copper is taken as anode whereas pure copper is taken as cathode.
Copper sulphate solution 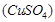 is taken as electrolyte. When electric current is passed, impure copper changes to ions which gain electrons at cathode and change into pure copper. Impurities are left behind as anode mud.
At anode
is taken as electrolyte. When electric current is passed, impure copper changes to ions which gain electrons at cathode and change into pure copper. Impurities are left behind as anode mud.
At anode 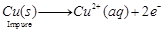 At cathode
At cathode 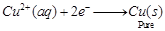
You need to login to perform this action.
You will be redirected in
3 sec
