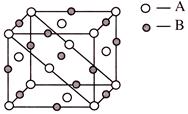
A) \[AB\]
B) \[{{A}_{5}}{{B}_{7}}\]
C) \[{{A}_{7}}{{B}_{5~}}\]
D) \[{{A}_{2}}{{B}_{3}}\]
Correct Answer: A
Solution :
\[{{A}_{\frac{4}{8}+\frac{4}{2}}}\] \[{{B}_{4-1-\frac{2}{4}}}\] \[{{A}_{2.5}}\,{{B}_{2.5}}\,or\,\,AB\]You need to login to perform this action.
You will be redirected in
3 sec
