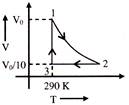
A) -59 J
B) -47J
C) -42 J
D) None of these
Correct Answer: B
Solution :
\[{{W}_{12}}\,=\,\,-\frac{nR({{T}_{1}}-{{T}_{2}})}{\gamma -1}\,\,=\,\,-\frac{R{{T}_{1}}({{10}^{\frac{\gamma -1}{\gamma }}}-1)}{\gamma -1}\] \[=\text{ }-\text{ }5600\text{ }kJ\] \[{{W}_{23}}=0\] \[{{W}_{31}}\,\,=\,\,nR{{T}_{1}}\text{ }\ell n\left( \frac{V}{V/10} \right)\,\,=\,\,R{{T}_{1}}\text{ }In\text{ }10\] \[=\text{ }5552.67\text{ }J\] \[\therefore \,\,\,\,\Delta Q\,\,=\,\,{{W}_{net}}=-\,47.33\text{ }J\]You need to login to perform this action.
You will be redirected in
3 sec
