A)

B)
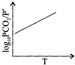
C)
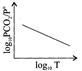
D)
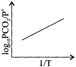
Correct Answer: A
Solution :
For the reaction \[{{\operatorname{K}}_{p}}={{P}_{C{{O}_{2}}}}\,\,or\,\,{{P}_{C{{O}_{2}}}}/P{}^\circ \] \[\operatorname{P}{}^\circ = reference pressure of 1 atm\] According to van't Hoff equation, \[\operatorname{d}\frac{\ell nkp}{dT}=\frac{\Delta {{H}^{o}}}{R{{T}^{2}}}\] \[\operatorname{In}\,\,{{K}_{p}}=\frac{-\Delta {{H}^{o}}}{RT}+\operatorname{I}\] \[\operatorname{Or} 2.303 log {{P}_{C{{O}_{2}}}} /P{}^\circ =\frac{\Delta {{H}^{o}}}{RT}+I\] Hence plot of log \[{{P}_{C{{O}_{2}}}} /P{}^\circ \] versus 1/T will be a straight line with a negative slope.You need to login to perform this action.
You will be redirected in
3 sec
