A) \[{{\operatorname{NO}}_{3}}^{-}\]
B) \[{{\operatorname{NO}}_{2}}^{-}\]
C) \[{{\operatorname{NO}}_{2}}\]
D) \[{{\operatorname{NO}}_{2}}^{+}\]
Correct Answer: D
Solution :
\[{{\operatorname{NO}}^{+}}_{2}\] \[{{\operatorname{NO}}^{-}}_{3}has s{{p}^{2}}\] hybridisation, resonating structure \[{{\operatorname{NO}}^{-}}_{2}\]has no unshared electron & has sp hybridisation shape is linear. \[(\underset{..}{\overset{..}{\mathop{o}}}\,\bar{-}N\bar{-}\underset{..}{\overset{..}{\mathop{o}}}\,) With bond angle = 180{}^\circ N{{O}_{2}} has\]one unshared electron. Whereas\[N{{O}_{2}}^{-}\]has one unshared electron pair. Hence in\[N{{O}_{2}}^{-}\]repulsion on bond pair are more and angle is less
\[{{\operatorname{NO}}^{-}}_{2}\]has no unshared electron & has sp hybridisation shape is linear. \[(\underset{..}{\overset{..}{\mathop{o}}}\,\bar{-}N\bar{-}\underset{..}{\overset{..}{\mathop{o}}}\,) With bond angle = 180{}^\circ N{{O}_{2}} has\]one unshared electron. Whereas\[N{{O}_{2}}^{-}\]has one unshared electron pair. Hence in\[N{{O}_{2}}^{-}\]repulsion on bond pair are more and angle is less 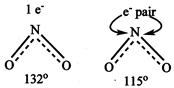
You need to login to perform this action.
You will be redirected in
3 sec
