A) 550J
B) 650J
C) 750J
D) 850J
Correct Answer: B
Solution :
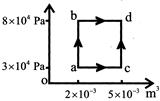
There is no volume change in process ab so \[\Rightarrow W = 0\] Process bd, occurs at constant pressure \[\Rightarrow W by system\] \[= P\left( {{V}_{2}} -{{V}_{1}} \right) = 8\times 1{{0}^{4}} \left( 5\times 1{{0}^{-3}} -2\times 1{{0}^{-3}} \right) = 240J\]\[{{\operatorname{W}}_{abd}}=240J,~~~~~{{Q}_{abd}}=800J\] \[\Delta U=Q-W=800-240 =560J\] \[\Delta U is state function\] \[{{\operatorname{W}}_{acd}}=3\times 1{{0}^{4}}\left( 5\times {{10}^{-3}}-2\times {{10}^{-3}} \right)=90J\] Total heat added in the path acd Q=AU+W Q = 560 + 90 = 650JYou need to login to perform this action.
You will be redirected in
3 sec
